Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2020-09-14 , DOI: 10.1016/j.bbamem.2020.183473 Lais Alonso 1 , Jéssica Carreira de Paula 2 , Paula Baréa 3 , Maria Helena Sarragiotto 3 , Tânia Ueda-Nakamura 2 , Antonio Alonso 4 , Nilma de Souza Fernandes 2 , César Armando Contreras Lancheros 2 , Hélito Volpato 2 , Danielle Lazarin-Bidóia 2 , Celso Vataru Nakamura 2
|
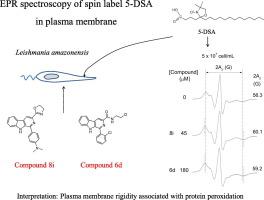
Two β-carboline compounds, 8i and 6d, demonstrated in vitro antileishmanial activity against Leishmania (L.) amazonensis promastigotes similar to that of miltefosine (MIL). Estimates of the membrane-water partition coefficient (KM/W) and the compound concentrations in the membrane (cm50) and aqueous phase (cw50) for half maximal inhibitory concentration were made. Whereas these biophysical parameters for 6d were not significantly different from those reported for MIL, 8i showed lower affinity for the parasite membrane (lower KM/W) and a lower concentration of the compound in the membrane required to inhibit the growth of the parasite (lower cm50). A 2-hour treatment of Leishmania promastigotes with the compounds 8i and 6d caused membrane rigidity in a concentration-dependent manner, as demonstrated by the electron paramagnetic resonance (EPR) technique and spin label method. This increased rigidity of the membrane was interpreted to be associated with the occurrence of cross-linking of oxidized cytoplasmic proteins to the parasite membrane skeleton. Importantly, the two β-carboline-oxazoline derivatives showed low hemolytic action, both in experiments with isolated red blood cells or with whole blood, denoting their great Leishmania/erythrocyte selectivity index. Using electron microscopy, changes in the membrane of both the amastigote and promastigote form of the parasite were confirmed, and it was demonstrated that compounds 8i and 6d decreased the number of amastigotes in infected murine macrophages. Furthermore, 8i and 6d were more toxic to the protozoa than to J774A.1 macrophages, with treated promastigotes exhibiting a decrease in cell volume, mitochondrial membrane potential depolarization, accumulation of lipid bodies, increased ROS production and changes in the cell cycle.
中文翻译:

亚马逊利什曼原虫的膜动力学和β-咔啉衍生物的抗疟疾活性。
两种β-咔啉化合物8i和6d具有类似于miltefosine (MIL)的体外对利什曼原虫(L.)Amazon amazonensis前鞭毛虫的体外抗疟原虫活性。估算了最大抑制浓度一半时的膜水分配系数(K M / W)和膜中化合物浓度(c m50)和水相(c w50)。尽管6d的这些生物物理参数与MIL报道的无明显差异,但8i对寄生虫膜的亲和力较低(K M / W较低)。)和抑制寄生虫生长所需的膜中较低浓度的化合物(较低的c m50)。化合物8i和6d对利什曼原虫前鞭毛体进行2小时的处理,会导致膜的硬度以浓度依赖性的方式出现,如电子顺磁共振(EPR)技术和自旋标记法所示。膜的这种增加的刚性被解释为与氧化的细胞质蛋白交联到寄生虫膜骨架上有关。重要的是,两种β-咔啉-恶唑啉衍生物在分离的红血球或全血的实验中均显示出低溶血作用,这表明它们的利什曼原虫/红细胞选择性指数。使用电子显微镜,证实了寄生虫的假阳具和前鞭毛体形式的膜的变化,并且证明了化合物8i和6d减少了感染的鼠巨噬细胞中的变形虫的数量。此外,8i和6d对原生动物的毒性大于对J774A.1巨噬细胞的毒性,处理过的前鞭毛体动物显示出细胞体积减少,线粒体膜电位去极化,脂质体积累,ROS产生增加以及细胞周期变化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号