当前位置:
X-MOL 学术
›
Chem. Heterocycl. Comp.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Generation and hydrolysis of N -acyloxazolinium salts allowing regiospecific acylation of chiral amino alcohols
Chemistry of Heterocyclic Compounds ( IF 1.4 ) Pub Date : 2020-06-02 , DOI: 10.1007/s10593-020-02707-3 R. Alan Aitken , Alexandra M. Z. Slawin , Andrew Wilson
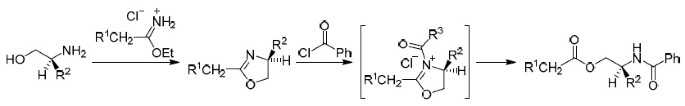
中文翻译:

N-酰基恶唑啉鎓盐的生成和水解可实现手性氨基醇的区域特异性酰化

Chemistry of Heterocyclic Compounds ( IF 1.4 ) Pub Date : 2020-06-02 , DOI: 10.1007/s10593-020-02707-3 R. Alan Aitken , Alexandra M. Z. Slawin , Andrew Wilson

中文翻译:

N-酰基恶唑啉鎓盐的生成和水解可实现手性氨基醇的区域特异性酰化












































 京公网安备 11010802027423号
京公网安备 11010802027423号