当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Conversion of Benzyl Alcohol to Benzaldehyde over a Cu-CeO2 Catalyst: Utilization of CO2 for Enhancement of Selectivity and Activity
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2020-09-13 , DOI: 10.1021/acs.iecr.0c02464 Prathap Challa 1, 2 , Siva Sankar Enumula 2 , Saidulu Reddy K 1, 2 , Murali Kondeboina 2 , David Raju Burri 2 , Seetha Rama Rao Kamaraju 2
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2020-09-13 , DOI: 10.1021/acs.iecr.0c02464 Prathap Challa 1, 2 , Siva Sankar Enumula 2 , Saidulu Reddy K 1, 2 , Murali Kondeboina 2 , David Raju Burri 2 , Seetha Rama Rao Kamaraju 2
Affiliation
|
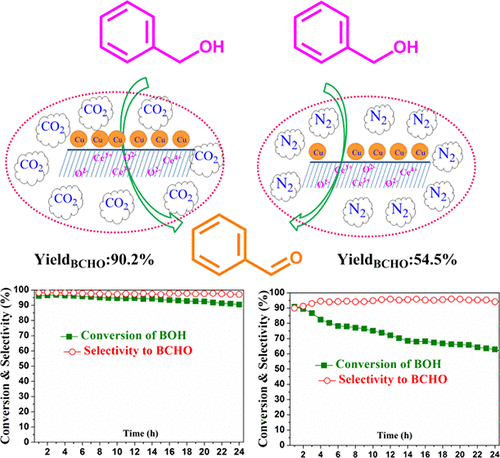
CO2-assisted dehydrogenation of benzyl alcohol to benzaldehyde over Cu nanoparticles dispersed on CeO2 was reported. Cu nanoparticles with an average size of ∼11.4 nm dispersed over CeO2 cubes were efficient in selective conversion of benzyl alcohol with a rate of formation of benzaldehyde of 250.99 μmol s–1 gcat–1. The high rate of reaction might be due to the miscibility of BOH in CO2, which led to enhanced diffusion of BOH reactant molecules toward active sites. The controlled surface acid–base sites were responsible for the activation of benzyl alcohol, and nearby Cu nanoparticles abstracted α-H of benzyl alcohol to form benzaldehyde. During a time on stream study, the Cu-CeO2 catalyst experienced a gradual deactivation in the presence of N2 as the carrier gas, while in the presence of CO2, it delivered constant activity for 24 h. In the presence of N2, in-situ generated hydrogen was responsible for the formation of much toluene via hydrogenolysis of benzyl alcohol. CO2 acted as a soft oxidant, which minimized the in-situ generated hydrogen via the reverse water gas shift reaction; as a result, the toluene formation and deposition of carbonaceous species were minimized.
中文翻译:

在Cu-CeO 2催化剂上将苄醇催化转化为苯甲醛:利用CO 2增强选择性和活性
报道了在分散在CeO 2上的Cu纳米颗粒上,CO 2辅助苄醇脱氢为苯甲醛。平均粒径约为11.4 nm的Cu纳米颗粒分散在CeO 2立方体上,可有效地选择性转化苄醇,苯甲醛的形成速率为250.99μmols –1 g cat –1。高反应速率可能是由于BOH在CO 2中的混溶性,这导致BOH反应物分子向活性位点的扩散增强。受控的表面酸碱位点负责苄醇的活化,附近的Cu纳米粒子提取了苄醇的α-H形成苯甲醛。在一段时间的研究中,Cu-CeO 2催化剂在以N 2为载气的情况下逐渐失活,而在CO 2的存在下,其活性持续24小时。在N 2的存在下,原位产生的氢通过苯甲醇的氢解作用导致大量甲苯的形成。一氧化碳2用作一种软氧化剂,可通过逆向水煤气变换反应将原位产生的氢气降至最低;结果,使碳质物质的甲苯形成和沉积最小化。
更新日期:2020-10-07
中文翻译:

在Cu-CeO 2催化剂上将苄醇催化转化为苯甲醛:利用CO 2增强选择性和活性
报道了在分散在CeO 2上的Cu纳米颗粒上,CO 2辅助苄醇脱氢为苯甲醛。平均粒径约为11.4 nm的Cu纳米颗粒分散在CeO 2立方体上,可有效地选择性转化苄醇,苯甲醛的形成速率为250.99μmols –1 g cat –1。高反应速率可能是由于BOH在CO 2中的混溶性,这导致BOH反应物分子向活性位点的扩散增强。受控的表面酸碱位点负责苄醇的活化,附近的Cu纳米粒子提取了苄醇的α-H形成苯甲醛。在一段时间的研究中,Cu-CeO 2催化剂在以N 2为载气的情况下逐渐失活,而在CO 2的存在下,其活性持续24小时。在N 2的存在下,原位产生的氢通过苯甲醇的氢解作用导致大量甲苯的形成。一氧化碳2用作一种软氧化剂,可通过逆向水煤气变换反应将原位产生的氢气降至最低;结果,使碳质物质的甲苯形成和沉积最小化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号