当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparative conventional and microwave assisted synthesis of heterocyclic oxadiazole analogues having enzymatic inhibition potential
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-09-13 , DOI: 10.1002/jhet.4150 Jamilah Javid 1 , Aziz‐ur‐ Rehman 1 , Muhammad A. Abbasi 1 , Sabahat Z. Siddiqui 1 , Javed Iqbal , Naeem A. Virk 1 , Shahid Rasool 1 , Hira A. Ali 1 , Muhammad Ashraf 2 , Wardah Shahid 2 , Safdar Hussain 2 , Syed A. Ali Shah 3, 4
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-09-13 , DOI: 10.1002/jhet.4150 Jamilah Javid 1 , Aziz‐ur‐ Rehman 1 , Muhammad A. Abbasi 1 , Sabahat Z. Siddiqui 1 , Javed Iqbal , Naeem A. Virk 1 , Shahid Rasool 1 , Hira A. Ali 1 , Muhammad Ashraf 2 , Wardah Shahid 2 , Safdar Hussain 2 , Syed A. Ali Shah 3, 4
Affiliation
|
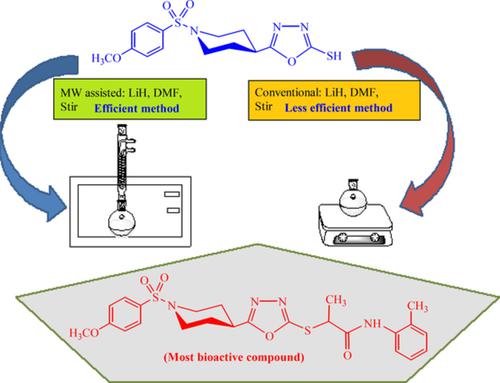
A comparative microwave assisted and conventional synthetic strategies were applied to synthesize heterocyclic 1,3,4‐oxadiazole analogues as active anti‐enzymatic agents. Green synthesis of compound 1 was achieved by stirring 4‐methoxybenzenesulfonyl chloride (a) and ethyl piperidine‐4‐carboxylate (b). Compound 1 was converted into respective hydrazide (2) by hydrazine and then into 1,3,4‐oxadiazole (3) by CS2 on reflux. The electrophiles, N‐alkyl/aralkyl/aryl‐2‐bromopropanamides (6a–p) were synthesized and converted to N‐alkyl/aralkyl/aryl‐2‐propanamide derivatives (7a–p) by reaction with 3 under green chemistry. Microwave assisted method was found to be effective relative to conventional method. 13C‐NMR, 1H‐NMR and IR techniques were availed to corroborate structures of synthesized compounds and then subjected to screening against lipoxygenase (LOX), α‐glucosidase, acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) enzymes. A number of compounds presented better potential against these enzymes. The most active compounds against LOX and α‐glucosidase enzymes were subjected to molecular docking study to explore their interactions with the active sites of the enzymes.
中文翻译:

比较常规和微波辅助合成具有酶抑制潜力的杂环恶二唑类似物
比较了微波辅助和常规合成策略,以合成杂环1,3,4-恶二唑类似物作为活性抗酶剂。通过搅拌4-甲氧基苯磺酰氯(a)和哌啶-4-羧酸乙酯(b)实现化合物1的绿色合成。化合物1通过肼分别转化为酰肼(2),然后在回流下通过CS 2转化为1,3,4-恶二唑(3)。合成了亲电子试剂N-烷基/芳烷基/芳基-2-溴丙酰胺(6a-p)并转化为N-烷基/芳烷基/芳基-2-丙酰胺衍生物(7a–p)通过在绿色化学反应下与3反应。发现微波辅助方法相对于常规方法是有效的。利用13 C-NMR,1 H-NMR和IR技术证实了合成化合物的结构,然后针对脂氧合酶(LOX),α-葡萄糖苷酶,乙酰胆碱酯酶(AChE)和丁酰胆碱酯酶(BChE)酶进行了筛选。许多化合物针对这些酶表现出更好的潜力。针对LOX和α-葡萄糖苷酶的活性最强的化合物进行了分子对接研究,以探索它们与酶活性位点的相互作用。
更新日期:2020-09-13
中文翻译:

比较常规和微波辅助合成具有酶抑制潜力的杂环恶二唑类似物
比较了微波辅助和常规合成策略,以合成杂环1,3,4-恶二唑类似物作为活性抗酶剂。通过搅拌4-甲氧基苯磺酰氯(a)和哌啶-4-羧酸乙酯(b)实现化合物1的绿色合成。化合物1通过肼分别转化为酰肼(2),然后在回流下通过CS 2转化为1,3,4-恶二唑(3)。合成了亲电子试剂N-烷基/芳烷基/芳基-2-溴丙酰胺(6a-p)并转化为N-烷基/芳烷基/芳基-2-丙酰胺衍生物(7a–p)通过在绿色化学反应下与3反应。发现微波辅助方法相对于常规方法是有效的。利用13 C-NMR,1 H-NMR和IR技术证实了合成化合物的结构,然后针对脂氧合酶(LOX),α-葡萄糖苷酶,乙酰胆碱酯酶(AChE)和丁酰胆碱酯酶(BChE)酶进行了筛选。许多化合物针对这些酶表现出更好的潜力。针对LOX和α-葡萄糖苷酶的活性最强的化合物进行了分子对接研究,以探索它们与酶活性位点的相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号