Sustainable Chemistry and Pharmacy ( IF 5.5 ) Pub Date : 2020-09-12 , DOI: 10.1016/j.scp.2020.100318 Paula Mayara Morais da Silva , Natália Gabriele Camparotto , Katherly Tainá Grego Lira , Carolina Siqueira Franco Picone , Patrícia Prediger
|
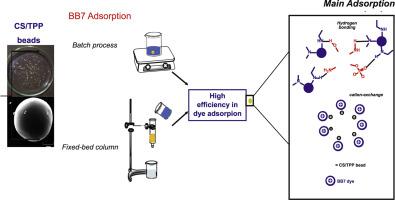
This study aims to develop sustainable low cost chitosan-based beads by a simple dropping method, with instantaneous formation by reticulation with tripolyphosphate anion. The beads were characterized and applied to basic blue 7 uptake. Adsorption process was optimized through variation of several parameters in batch experiments such as beads loading, pH, dye concentration and temperature. The best BB7 adsorption capacity by chitosan-beads was achieved in 60 min (1174 mg/g), which was quite superior to others described in the literature. Kinetics data could be better fitted to pseudo-first order and the adsorption process was better described by the Langmuir type model, MX subclass. The thermodynamic parameters calculated suggested that the adsorption process is exothermic and favorable. The beads storage study indicated that for a two-week period their adsorption capacity was not affected. Also, the presence of interfering agents did not harmed basic blue 7 removal, especially in saline solution. In addition, in the continuous-mode it was achieved 100% of dye uptake in the initial 2h. Furthermore, the removal mechanism study suggests that hydrogen bounds are the main interaction between chitosan beads and the dye. This study gave rise to encouraging results and sustainable chitosan/tripolyphosphate beads proved to be potential adsorbents for industrial effluent treatments, although in the presence of other dyes and saline medium. This procedure allows wastewater to be reused for further applications.
中文翻译:

碱性壳聚糖珠粒上碱性染料的吸附去除:平衡,动力学,稳定性,连续模式吸附及其机理
这项研究旨在通过一种简单的滴加方法开发可持续的,低成本的壳聚糖基微珠,并通过与三聚磷酸根阴离子的网状化而瞬时形成。对珠子进行表征并应用于碱性蓝7摄取。通过分批实验中几个参数的变化来优化吸附过程,例如珠负载,pH,染料浓度和温度。壳聚糖微珠在60分钟内(1174 mg / g)获得了最佳的BB7吸附能力,这远远优于文献中描述的其他能力。动力学数据可以更好地拟合拟一级,吸附过程可以用Langmuir型模型MX子类更好地描述。计算得到的热力学参数表明吸附过程是放热的并且是有利的。磁珠存储研究表明,在两周的时间内它们的吸附能力没有受到影响。而且,干扰剂的存在不损害碱性蓝7的去除,尤其是在盐溶液中。另外,在连续模式下,在最初的2小时内达到100%的染料吸收率。此外,去除机理研究表明,氢键是壳聚糖珠和染料之间的主要相互作用。这项研究产生了令人鼓舞的结果,可持续的壳聚糖/三聚磷酸酯珠被证明是工业废水处理的潜在吸附剂,尽管存在其他染料和盐水介质。该程序可将废水再利用以进一步应用。特别是在盐溶液中。另外,在连续模式下,在最初的2小时内达到100%的染料吸收率。此外,去除机理研究表明,氢键是壳聚糖珠和染料之间的主要相互作用。这项研究产生了令人鼓舞的结果,可持续的壳聚糖/三聚磷酸酯珠被证明是工业废水处理的潜在吸附剂,尽管存在其他染料和盐水介质。该程序允许废水被再利用以进一步应用。特别是在盐溶液中。另外,在连续模式下,在最初的2小时内达到100%的染料吸收率。此外,去除机理研究表明,氢键是壳聚糖珠和染料之间的主要相互作用。这项研究产生了令人鼓舞的结果,可持续的壳聚糖/三聚磷酸酯珠被证明是工业废水处理的潜在吸附剂,尽管存在其他染料和盐水介质。该程序允许废水被再利用以进一步应用。这项研究产生了令人鼓舞的结果,可持续的壳聚糖/三聚磷酸酯珠被证明是工业废水处理的潜在吸附剂,尽管存在其他染料和盐水介质。该程序允许废水被再利用以进一步应用。这项研究产生了令人鼓舞的结果,可持续的壳聚糖/三聚磷酸酯珠被证明是工业废水处理的潜在吸附剂,尽管存在其他染料和盐水介质。该程序允许废水被再利用以进一步应用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号