Journal of Inorganic Biochemistry ( IF 3.9 ) Pub Date : 2020-09-12 , DOI: 10.1016/j.jinorgbio.2020.111254 Celine Eidenschenk 1 , Lionel Cheruzel 2
|
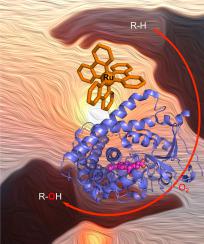
With a growing interest in utilizing visible light to drive biocatalytic processes, several light-harvesting units and approaches have been employed to harness the synthetic potential of heme monooxygenases and carry out selective oxyfunctionalization of a wide range of substrates. While the fields of cytochrome P450 and Ru(II) photochemistry have separately been prolific, it is not until the turn of the 21st century that they converged. Non-covalent and subsequently covalently attached Ru(II) complexes were used to promote rapid intramolecular electron transfer in bacterial P450 enzymes. Photocatalytic activity with Ru(II)-modified P450 enzymes was achieved under reductive conditions with a judicious choice of a sacrificial electron donor. The initial concept of Ru(II)-modified P450 enzymes was further improved using protein engineering, photosensitizer functionalization and was successfully applied to other P450 enzymes. In this review, we wish to present the recent contributions from our group and others in utilizing Ru(II) complexes coupled with P450 enzymes in the broad context of photobiocatalysis, protein assemblies and chemoenzymatic reactions. The merging of chemical catalysts with the synthetic potential of P450 enzymes has led to the development of several chemoenzymatic approaches. Moreover, strained Ru(II) compounds have been shown to selectively inhibit P450 enzymes by releasing aromatic heterocycle containing molecules upon visible light excitation taking advantage of the rapid ligand loss feature in those complexes.
中文翻译:

Ru(II)-二亚胺配合物和细胞色素P450协同工作
随着对利用可见光来驱动生物催化过程的兴趣日益浓厚,已采用了几种光收集单元和方法来利用血红素单加氧酶的合成潜力,并对各种底物进行选择性的氧官能化。虽然细胞色素P450和Ru(II)光化学领域分别是多产的,但直到21世纪初,它们才趋于融合。非共价和随后共价连接的Ru(II)复合物用于促进细菌P450酶中分子内电子的快速转移。明智地选择牺牲电子供体,在还原条件下实现了Ru(II)修饰的P450酶的光催化活性。Ru(II)修饰的P450酶的最初概念通过蛋白质工程得到了进一步改进,光敏剂功能化并成功应用于其他P450酶。在这篇综述中,我们希望介绍在光生物催化,蛋白质组装和化学酶反应的广泛背景下,我们小组和其他人在利用Ru(II)配合物与P450酶结合方面的最新贡献。化学催化剂与P450酶的合成潜力的合并导致了几种化学酶方法的发展。此外,已显示出应变的Ru(II)化合物可利用可见光激发下的配体快速损失特征,通过在可见光激发下释放含芳香族杂环的分子来选择性抑制P450酶。我们希望介绍我们小组和其他人最近在光生物催化,蛋白质组装和化学酶反应的广泛背景下利用Ru(II)配合物与P450酶结合的最新贡献。化学催化剂与P450酶的合成潜力的合并导致了几种化学酶方法的发展。此外,已显示出应变的Ru(II)化合物可利用可见光激发下的配体快速损失特征,通过在可见光激发下释放含芳香族杂环的分子来选择性抑制P450酶。我们希望介绍我们小组和其他人最近在光生物催化,蛋白质组装和化学酶反应的广泛背景下利用Ru(II)配合物与P450酶结合的最新贡献。化学催化剂与P450酶的合成潜力的合并导致了几种化学酶方法的发展。此外,已显示出应变的Ru(II)化合物可利用可见光激发下的配体快速损失特征,通过在可见光激发下释放含芳香族杂环的分子来选择性抑制P450酶。化学催化剂与P450酶的合成潜力的合并导致了几种化学酶学方法的发展。此外,已显示出应变的Ru(II)化合物可利用可见光激发下的配体快速损失特征,通过在可见光激发下释放含芳香族杂环的分子来选择性抑制P450酶。化学催化剂与P450酶的合成潜力的合并导致了几种化学酶方法的发展。此外,已显示出应变的Ru(II)化合物可利用可见光激发下的配体快速损失特征,通过在可见光激发下释放含芳香族杂环的分子来选择性抑制P450酶。




























 京公网安备 11010802027423号
京公网安备 11010802027423号