Applied Catalysis A: General ( IF 4.7 ) Pub Date : 2020-09-13 , DOI: 10.1016/j.apcata.2020.117812 Kaori Omata , Tomonori Nambu
|
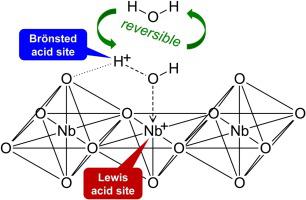
The effects of water addition on cumene cracking over niobium oxide and H-ZSM-5 are investigated in order to clarify the behavior of water molecules on solid acid catalysts. The activity of niobium oxide was improved in the presence of water vapor although H-ZSM-5 showed constant activity. When heavy water was introduced into the reaction over niobium oxide, the hydrogen atoms were substituted with the deuterium atoms in about 85 % of the produced benzene. The heavy water molecule on niobium oxide acts as the catalyst for cumene cracking, resulting in the formation of benzene containing D atoms. In addition, the Lewis acid on the niobium oxide was observed to change reversibly to the Brönsted acid in the presence of water using IR of adsorbed pyridine. We propose a mechanism that water behaves as Brönsted acid, which was clarified by repeating adsorption and desorption on Lewis acid sites of niobium oxide.
中文翻译:

氧化铌路易斯酸位点上作为布朗斯台德酸的水分子的催化作用
为了阐明水分子在固体酸催化剂上的行为,研究了加水对氧化铌和H-ZSM-5上枯烯裂化的影响。尽管H-ZSM-5表现出恒定的活性,但是在水蒸气存在下氧化铌的活性得到了改善。当将重水通过氧化铌引入反应中时,约85%的生成苯中的氢原子被氘原子取代。氧化铌上的重水分子充当枯烯裂化的催化剂,导致形成含D原子的苯。另外,观察到氧化铌上的路易斯酸在存在水的情况下使用吸附的吡啶的IR可逆地变为布朗斯台德酸。我们提出一种机制,使水表现为布朗斯台德酸,











































 京公网安备 11010802027423号
京公网安备 11010802027423号