当前位置:
X-MOL 学术
›
J. Neurochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Disorders of synaptic vesicle fusion machinery
Journal of Neurochemistry ( IF 4.2 ) Pub Date : 2020-09-11 , DOI: 10.1111/jnc.15181 Holly Melland 1 , Elyas H Arvell 1 , Sarah L Gordon 1
Journal of Neurochemistry ( IF 4.2 ) Pub Date : 2020-09-11 , DOI: 10.1111/jnc.15181 Holly Melland 1 , Elyas H Arvell 1 , Sarah L Gordon 1
Affiliation
|
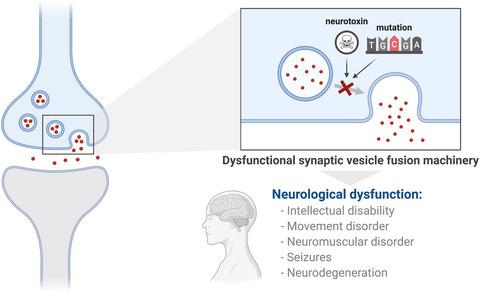
The revolution in genetic technology has ushered in a new age for our understanding of the underlying causes of neurodevelopmental, neuromuscular and neurodegenerative disorders, revealing that the presynaptic machinery governing synaptic vesicle fusion is compromised in many of these neurological disorders. This builds upon decades of research showing that disturbance to neurotransmitter release via toxins can cause acute neurological dysfunction. In this review, we focus on disorders of synaptic vesicle fusion caused either by toxic insult to the presynapse or alterations to genes encoding the key proteins that control and regulate fusion: the SNARE proteins (synaptobrevin, syntaxin‐1 and SNAP‐25), Munc18, Munc13, synaptotagmin, complexin, CSPα, α‐synuclein, PRRT2 and tomosyn. We discuss the roles of these proteins and the cellular and molecular mechanisms underpinning neurological deficits in these disorders.
中文翻译:

突触小泡融合机制障碍
基因技术的革命为我们理解神经发育、神经肌肉和神经退行性疾病的根本原因开辟了一个新时代,揭示了在许多这些神经系统疾病中控制突触小泡融合的突触前机制受到损害。这是建立在数十年研究的基础上的,研究表明通过毒素干扰神经递质释放会导致急性神经功能障碍。在这篇综述中,我们重点关注由突触前毒性损伤或编码控制和调节融合的关键蛋白的基因改变引起的突触小泡融合障碍:SNARE 蛋白(synaptobrevin、syntaxin-1 和 SNAP-25)、Munc18 、Munc13、突触结合蛋白、络合物、CSPα、α-突触核蛋白、PRRT2 和 tomosyn。
更新日期:2020-09-11
中文翻译:

突触小泡融合机制障碍
基因技术的革命为我们理解神经发育、神经肌肉和神经退行性疾病的根本原因开辟了一个新时代,揭示了在许多这些神经系统疾病中控制突触小泡融合的突触前机制受到损害。这是建立在数十年研究的基础上的,研究表明通过毒素干扰神经递质释放会导致急性神经功能障碍。在这篇综述中,我们重点关注由突触前毒性损伤或编码控制和调节融合的关键蛋白的基因改变引起的突触小泡融合障碍:SNARE 蛋白(synaptobrevin、syntaxin-1 和 SNAP-25)、Munc18 、Munc13、突触结合蛋白、络合物、CSPα、α-突触核蛋白、PRRT2 和 tomosyn。









































 京公网安备 11010802027423号
京公网安备 11010802027423号