当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rhodium-catalyzed [4+1] annulation of sulfoxonium ylides: Sequential ortho-CH functionalization/carbonyl α-amination toward polycyclic quinazolinones
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-09-12 , DOI: 10.1016/j.tetlet.2020.152441 Chang Wang , Peng-Cheng Qian , Fan Chen , Jiang Cheng
中文翻译:

铑催化的亚砜基鎓盐的[4 + 1]环合反应:顺式-C H官能化/羰基α-胺化成多环喹唑啉酮
H官能化/羰基α-胺化成多环喹唑啉酮
更新日期:2020-10-06
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-09-12 , DOI: 10.1016/j.tetlet.2020.152441 Chang Wang , Peng-Cheng Qian , Fan Chen , Jiang Cheng
|
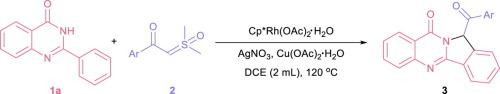
A rhodium-catalyzed [4+1] annulation between 2-aryl quinazolin-4(3H)-ones and sulfoxonium ylides was developed, leading to 12-aroyl isoindolo[1,2-b]quinazolin-10(12H)-ones in moderate to good yields. This reaction proceeds with sequential ortho-C H functionalization of 2-aryl quinazolin-4(3H)-ones and an intramolecular rhodium-catalyzed α-amination of carbonyl to furnish the cyclization. This procedure was applied to access some 13-aroyl luotonin A derivatives whereby the direct construction of ring c.
H functionalization of 2-aryl quinazolin-4(3H)-ones and an intramolecular rhodium-catalyzed α-amination of carbonyl to furnish the cyclization. This procedure was applied to access some 13-aroyl luotonin A derivatives whereby the direct construction of ring c.
中文翻译:

铑催化的亚砜基鎓盐的[4 + 1]环合反应:顺式-C
 H官能化/羰基α-胺化成多环喹唑啉酮
H官能化/羰基α-胺化成多环喹唑啉酮
开发了铑催化的2-芳基喹唑啉-4(3 H)-酮与亚砜基砜之间的[4 + 1]环化反应,从而制得12-芳基异吲哚并[1,2 - b ]喹唑啉-10(12 H)-中等至高产的玉米。该反应以2-芳基喹唑啉-4(3H)-的顺序邻位C  H官能化和分子内铑催化的羰基α-氨基化以提供环化作用而进行。将该方法用于获得一些13-芳酰基荧光素A衍生物,由此直接构建环c。
H官能化和分子内铑催化的羰基α-氨基化以提供环化作用而进行。将该方法用于获得一些13-芳酰基荧光素A衍生物,由此直接构建环c。









































 京公网安备 11010802027423号
京公网安备 11010802027423号