当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fit-for-purpose synthesis of dual leucine zipper kinase (DLK) inhibitor GNE-834
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-09-12 , DOI: 10.1016/j.tetlet.2020.152430 Jie Xu , Diane E. Carrera , Danial Beaudry , Michael Siu , Malcolm P. Huestis , Wendy Liu , Rémy Angelaud , Francis Gosselin
中文翻译:

适合合成的双亮氨酸拉链激酶(DLK)抑制剂GNE-834
更新日期:2020-10-06
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-09-12 , DOI: 10.1016/j.tetlet.2020.152430 Jie Xu , Diane E. Carrera , Danial Beaudry , Michael Siu , Malcolm P. Huestis , Wendy Liu , Rémy Angelaud , Francis Gosselin
|
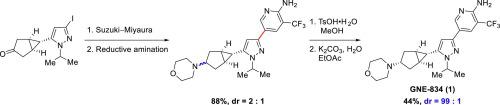
A practical fit-for-purpose synthesis of dual leucine zipper kinase (DLK) inhibitor GNE-834 (1) was developed. The key C C bond was constructed via a Suzuki–Miyaura cross-coupling of iodopyrazole 2 and pyridine boronic ester 3 to afford ketone 12. Subsequent selective reductive amination of ketone 12 with morpholine followed by a resolution via tosylate salt formation provided GNE-834 (1) in 39% overall yield from iodide 2 with 98.9 A% HPLC purity.
C bond was constructed via a Suzuki–Miyaura cross-coupling of iodopyrazole 2 and pyridine boronic ester 3 to afford ketone 12. Subsequent selective reductive amination of ketone 12 with morpholine followed by a resolution via tosylate salt formation provided GNE-834 (1) in 39% overall yield from iodide 2 with 98.9 A% HPLC purity.
中文翻译:

适合合成的双亮氨酸拉链激酶(DLK)抑制剂GNE-834
开发了一种实用的双亮氨酸拉链激酶(DLK)抑制剂GNE-834(1)的合成方法。关键的C  C键是通过Suzuki-Miyaura的碘吡唑2和吡啶硼酸酯3的交叉偶联而得到的,从而得到酮12。随后用吗啉对酮12进行选择性还原胺化,然后通过甲苯磺酸盐的形成进行拆分,从而提供了GNE-834(1),其碘化物2的总收率为39%,HPLC纯度为98.9 A%。
C键是通过Suzuki-Miyaura的碘吡唑2和吡啶硼酸酯3的交叉偶联而得到的,从而得到酮12。随后用吗啉对酮12进行选择性还原胺化,然后通过甲苯磺酸盐的形成进行拆分,从而提供了GNE-834(1),其碘化物2的总收率为39%,HPLC纯度为98.9 A%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号