当前位置:
X-MOL 学术
›
Process Saf. Environ. Prot.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evaluating the effect of multiple flammable gases on the flammability limit of CH4: Experimental study and theoretical calculation
Process Safety and Environmental Protection ( IF 6.9 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.psep.2020.09.023 Zhenmin Luo , He Liang , Tao Wang , Fangming Cheng , Bin Su , Litao Liu , Bo Liu
Process Safety and Environmental Protection ( IF 6.9 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.psep.2020.09.023 Zhenmin Luo , He Liang , Tao Wang , Fangming Cheng , Bin Su , Litao Liu , Bo Liu
|
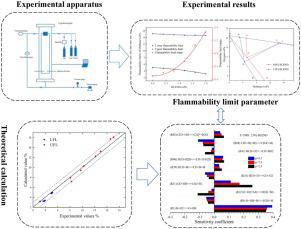
Abstract As the main component of natural gas and an important chemical raw material, methane easily forms a mixture of flammable gases during production and processing. Therefore, understanding the flammability limit and oxygen concentration limit of methane is very important in predicting the possibility of fire and explosion in industrial production and designing the corresponding monitoring parameters. To solve this problem, the effects of the addition of a C2H6/C2H4/CO/H2 mixture on the flammability limit of methane and limiting the oxygen concentration under N2 dilution were systematically studied. In addition, the flammability limit was evaluated using the limiting burning velocity theory and detailed reaction kinetic model, and a sensitivity analysis was conducted to study the chemical kinetics near the limit. The experimental results show that when the volume fraction of mixed gas gradually increases, the upper and lower flammability limit of methane both decrease, and the decrease in the lower flammability limit is larger than that of the upper flammability limit. Under the condition of nitrogen dilution, the limiting oxygen concentration gradually decreases with an increase in mixed gas concentration. The explosion triangle expands and moves to the lower-left corner, the flammable limit range increases, and a greater danger of explosion exists. The calculated results based on the limit laminar burning velocity are in good agreement with the experimental data. The sensitivity analysis shows that elementary reactions involving the active free radicals of OH, H and O have higher sensitivity. With the addition of mixed gas, the chain-branching reaction R31 is promoted, the chain termination reactions R12 and R41 are inhibited, the competition between the chain-branching reaction and chain termination reaction changes, and the flammable limit range of methane expands.
中文翻译:

评估多种可燃气体对 CH4 可燃性限值的影响:实验研究和理论计算
摘要 作为天然气的主要成分和重要的化工原料,甲烷在生产和加工过程中极易形成可燃气体混合物。因此,了解甲烷的可燃极限和氧浓度极限对于预测工业生产中发生火灾和爆炸的可能性以及设计相应的监测参数非常重要。为了解决这个问题,系统地研究了添加 C2H6/C2H4/CO/H2 混合物对甲烷可燃性极限和限制 N2 稀释下氧气浓度的影响。此外,使用极限燃烧速度理论和详细的反应动力学模型评估可燃极限,并进行敏感性分析以研究极限附近的化学动力学。实验结果表明,当混合气体体积分数逐渐增加时,甲烷的燃烧上下限均降低,且燃烧下限的降低幅度大于燃烧上限的降低幅度。在氮气稀释条件下,极限氧浓度随着混合气体浓度的增加而逐渐降低。爆炸三角形扩大并向左下角移动,可燃极限范围增大,存在更大的爆炸危险。基于极限层流燃烧速度的计算结果与实验数据吻合较好。敏感性分析表明,涉及OH、H和O活性自由基的基元反应具有较高的敏感性。随着混合气体的加入,
更新日期:2021-02-01
中文翻译:

评估多种可燃气体对 CH4 可燃性限值的影响:实验研究和理论计算
摘要 作为天然气的主要成分和重要的化工原料,甲烷在生产和加工过程中极易形成可燃气体混合物。因此,了解甲烷的可燃极限和氧浓度极限对于预测工业生产中发生火灾和爆炸的可能性以及设计相应的监测参数非常重要。为了解决这个问题,系统地研究了添加 C2H6/C2H4/CO/H2 混合物对甲烷可燃性极限和限制 N2 稀释下氧气浓度的影响。此外,使用极限燃烧速度理论和详细的反应动力学模型评估可燃极限,并进行敏感性分析以研究极限附近的化学动力学。实验结果表明,当混合气体体积分数逐渐增加时,甲烷的燃烧上下限均降低,且燃烧下限的降低幅度大于燃烧上限的降低幅度。在氮气稀释条件下,极限氧浓度随着混合气体浓度的增加而逐渐降低。爆炸三角形扩大并向左下角移动,可燃极限范围增大,存在更大的爆炸危险。基于极限层流燃烧速度的计算结果与实验数据吻合较好。敏感性分析表明,涉及OH、H和O活性自由基的基元反应具有较高的敏感性。随着混合气体的加入,











































 京公网安备 11010802027423号
京公网安备 11010802027423号