当前位置:
X-MOL 学术
›
Diam. Relat. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Zr(IV) functionalized Graphene oxide anchored sand as potential and economic adsorbent for fluoride removal from water
Diamond and Related Materials ( IF 4.3 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.diamond.2020.108081 C. Prathibha , Anjana Biswas , L.A. Avinash Chunduri , Shiva Konda Reddy , Paripurnanda Loganathan , Mahatheva Kalaruban , Kamisetti Venkatarmaniah
Diamond and Related Materials ( IF 4.3 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.diamond.2020.108081 C. Prathibha , Anjana Biswas , L.A. Avinash Chunduri , Shiva Konda Reddy , Paripurnanda Loganathan , Mahatheva Kalaruban , Kamisetti Venkatarmaniah
|
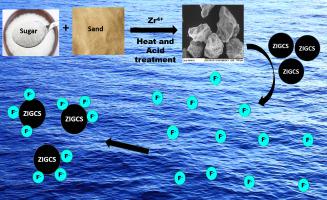
Abstract Excessive fluoride (F) in drinking water is a major problem affecting human health in many parts of the world. Cost-effective adsorbents are required for the defluoridation of drinking water. A carbon-based adsorbent, graphene oxide coated sand was produced from very inexpensive materials, sugar and river sand and impregnated with zirconium (Zr) for defluoridation of water. F adsorption by Zr impregnated graphene oxide coated sand (ZIGCS) at pH 4.0 satisfactorily fitted to Langmuir and Freundlich adsorption models with a Langmuir maximum adsorption capacity of 6.12 mg/g which is one of the highest values among the other carbon-based economic adsorbents reported for defluoridation. The adsorption of F on to ZIGCS (point of zero charge of pH 4.5) increased from pH 2 to 4 and then decreased up to 12. However, considerable adsorption capacity was observed throughout this pH range. Pseudo-second order model successfully described the adsorption reaction kinetics. Fluoride adsorbed on to ZIGCS was effectively desorbed using 0.1 M NaOH and the regenerated adsorbent maintained approximately 75% of the original F adsorption capacity even after five regeneration cycles. Thermodynamic data revealed that the adsorption process was endothermic and spontaneous with increase in randomness at the solid/solution interface.
中文翻译:

Zr(IV) 功能化氧化石墨烯锚定砂作为去除水中氟化物的潜在且经济的吸附剂
摘要 饮用水中过量的氟 (F) 是世界许多地区影响人类健康的主要问题。饮用水的除氟需要具有成本效益的吸附剂。一种碳基吸附剂、氧化石墨烯涂层沙子由非常便宜的材料、糖和河沙制成,并用锆 (Zr) 浸渍以对水进行除氟。Zr 浸渍的氧化石墨烯涂层砂 (ZIGCS) 在 pH 4.0 下的 F 吸附符合 Langmuir 和 Freundlich 吸附模型,Langmuir 最大吸附容量为 6.12 mg/g,这是其他碳基经济吸附剂中最高值之一用于脱氟。F 在 ZIGCS 上的吸附(pH 4.5 的零电荷点)从 pH 2 增加到 4,然后下降到 12。然而,在整个 pH 范围内观察到相当大的吸附能力。伪二级模型成功地描述了吸附反应动力学。吸附在 ZIGCS 上的氟化物可以使用 0.1 M NaOH 有效解吸,即使经过五次再生循环,再生后的吸附剂仍保持约 75% 的原始 F 吸附容量。热力学数据表明吸附过程是吸热和自发的,固/溶液界面的随机性增加。
更新日期:2020-11-01
中文翻译:

Zr(IV) 功能化氧化石墨烯锚定砂作为去除水中氟化物的潜在且经济的吸附剂
摘要 饮用水中过量的氟 (F) 是世界许多地区影响人类健康的主要问题。饮用水的除氟需要具有成本效益的吸附剂。一种碳基吸附剂、氧化石墨烯涂层沙子由非常便宜的材料、糖和河沙制成,并用锆 (Zr) 浸渍以对水进行除氟。Zr 浸渍的氧化石墨烯涂层砂 (ZIGCS) 在 pH 4.0 下的 F 吸附符合 Langmuir 和 Freundlich 吸附模型,Langmuir 最大吸附容量为 6.12 mg/g,这是其他碳基经济吸附剂中最高值之一用于脱氟。F 在 ZIGCS 上的吸附(pH 4.5 的零电荷点)从 pH 2 增加到 4,然后下降到 12。然而,在整个 pH 范围内观察到相当大的吸附能力。伪二级模型成功地描述了吸附反应动力学。吸附在 ZIGCS 上的氟化物可以使用 0.1 M NaOH 有效解吸,即使经过五次再生循环,再生后的吸附剂仍保持约 75% 的原始 F 吸附容量。热力学数据表明吸附过程是吸热和自发的,固/溶液界面的随机性增加。











































 京公网安备 11010802027423号
京公网安备 11010802027423号