当前位置:
X-MOL 学术
›
Colloids Surf. A Physicochem. Eng. Aspects
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of alkyl-functionalized magnetic for fluoroquinolones removal: Adsorption performance and mechanism studies in single and binary systems
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 4.9 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.colsurfa.2020.125549 Chun Yang , Rui Li , Qi Wang , Wenhao Wang , Pei Gao , Bibo Hu
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 4.9 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.colsurfa.2020.125549 Chun Yang , Rui Li , Qi Wang , Wenhao Wang , Pei Gao , Bibo Hu
|
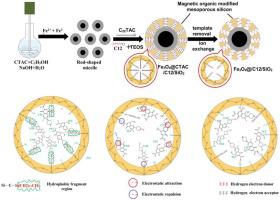
Abstract The functionalized mesoporous silica (FMS) and core-shell magnetic functionalized mesoporous silica (MMS) were synthesized on the basis of unfunctionalized mesoporous silica (UMS) via alkyl modification and one-pot reaction. Its adsorption properties to enrofloxacin (ENR), pefloxacin (PEF) and ciprofloxacin (CIP) were systematically investigated in single and binary systems. The results indicated the adsorption performance of MMS is five times higher than UMS. Batch adsorption experiments demonstrated the MMS had a higher adsorption capacity and adsorption efficiency for FQs, with the maximum adsorption capacities of 201.52 mg g-1 for CIP, 275.46 mg g-1 for PEF and 286.35 mg g-1 for ENR. Meanwhile, over 80% and 90% removals were achieved within 5 min and 10 min. Experiment results also presented that: MMS was able to reach the maximum adsorption capacity around pH 7.0 and maintain high adsorption capacity at a high concentration of humic acid. Besides, MMS showed the selective adsorption to three FQs in the binary adsorption experiments. The reusability experiment suggested the MMS possess excellent stability. H-bonding, electrostatic interaction and hydrophobic interaction were the main driving force between three FQs and MMS. Moreover, the adsorbent can be readily separated from water solution by an external magnet. These obtained results could be used for adsorbent design in considering of the properties of the adsorbates and the reuse of adsorbents.
中文翻译:

用于氟喹诺酮类去除的烷基官能化磁性材料的合成:单一和二元系统的吸附性能和机理研究
摘要 在未功能化介孔二氧化硅(UMS)的基础上,通过烷基改性和一锅法反应合成了功能化介孔二氧化硅(FMS)和核壳磁性功能化介孔二氧化硅(MMS)。在单一和二元系统中系统地研究了其对恩诺沙星 (ENR)、培氟沙星 (PEF) 和环丙沙星 (CIP) 的吸附性能。结果表明MMS的吸附性能是UMS的5倍。批量吸附实验表明,MMS 对 FQs 具有更高的吸附容量和吸附效率,CIP 的最大吸附容量为 201.52 mg g-1,PEF 为 275.46 mg g-1,ENR 为 286.35 mg g-1。同时,在 5 分钟和 10 分钟内实现了超过 80% 和 90% 的去除。实验结果还表明:MMS 能够在 pH 7.0 附近达到最大吸附容量,并在高浓度腐殖酸下保持高吸附容量。此外,MMS 在二元吸附实验中显示出对三种 FQ 的选择性吸附。可重用性实验表明 MMS 具有出色的稳定性。氢键、静电相互作用和疏水相互作用是三种 FQ 和 MMS 之间的主要驱动力。此外,吸附剂可以很容易地通过外部磁铁从水溶液中分离出来。考虑到吸附物的性质和吸附剂的再利用,这些获得的结果可用于吸附剂设计。MMS 在二元吸附实验中显示出对三种 FQ 的选择性吸附。可重用性实验表明 MMS 具有出色的稳定性。氢键、静电相互作用和疏水相互作用是三种 FQ 和 MMS 之间的主要驱动力。此外,吸附剂可以很容易地通过外部磁铁从水溶液中分离出来。考虑到吸附物的性质和吸附剂的再利用,这些获得的结果可用于吸附剂设计。MMS 在二元吸附实验中显示出对三种 FQ 的选择性吸附。可重用性实验表明 MMS 具有出色的稳定性。氢键、静电相互作用和疏水相互作用是三种 FQ 和 MMS 之间的主要驱动力。此外,吸附剂可以很容易地通过外部磁铁从水溶液中分离出来。考虑到吸附物的性质和吸附剂的再利用,这些获得的结果可用于吸附剂设计。
更新日期:2021-01-01
中文翻译:

用于氟喹诺酮类去除的烷基官能化磁性材料的合成:单一和二元系统的吸附性能和机理研究
摘要 在未功能化介孔二氧化硅(UMS)的基础上,通过烷基改性和一锅法反应合成了功能化介孔二氧化硅(FMS)和核壳磁性功能化介孔二氧化硅(MMS)。在单一和二元系统中系统地研究了其对恩诺沙星 (ENR)、培氟沙星 (PEF) 和环丙沙星 (CIP) 的吸附性能。结果表明MMS的吸附性能是UMS的5倍。批量吸附实验表明,MMS 对 FQs 具有更高的吸附容量和吸附效率,CIP 的最大吸附容量为 201.52 mg g-1,PEF 为 275.46 mg g-1,ENR 为 286.35 mg g-1。同时,在 5 分钟和 10 分钟内实现了超过 80% 和 90% 的去除。实验结果还表明:MMS 能够在 pH 7.0 附近达到最大吸附容量,并在高浓度腐殖酸下保持高吸附容量。此外,MMS 在二元吸附实验中显示出对三种 FQ 的选择性吸附。可重用性实验表明 MMS 具有出色的稳定性。氢键、静电相互作用和疏水相互作用是三种 FQ 和 MMS 之间的主要驱动力。此外,吸附剂可以很容易地通过外部磁铁从水溶液中分离出来。考虑到吸附物的性质和吸附剂的再利用,这些获得的结果可用于吸附剂设计。MMS 在二元吸附实验中显示出对三种 FQ 的选择性吸附。可重用性实验表明 MMS 具有出色的稳定性。氢键、静电相互作用和疏水相互作用是三种 FQ 和 MMS 之间的主要驱动力。此外,吸附剂可以很容易地通过外部磁铁从水溶液中分离出来。考虑到吸附物的性质和吸附剂的再利用,这些获得的结果可用于吸附剂设计。MMS 在二元吸附实验中显示出对三种 FQ 的选择性吸附。可重用性实验表明 MMS 具有出色的稳定性。氢键、静电相互作用和疏水相互作用是三种 FQ 和 MMS 之间的主要驱动力。此外,吸附剂可以很容易地通过外部磁铁从水溶液中分离出来。考虑到吸附物的性质和吸附剂的再利用,这些获得的结果可用于吸附剂设计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号