Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2020-09-12 , DOI: 10.1016/j.bmcl.2020.127550 Sivappa Rasapalli 1 , Zachary F Murphy 1 , Vamshikrishna Reddy Sammeta 1 , James A Golen 1 , Alexander W Weig 2 , Roberta J Melander 2 , Christian Melander 2 , Prathyushakrishna Macha 3 , Milana C Vasudev 3
|
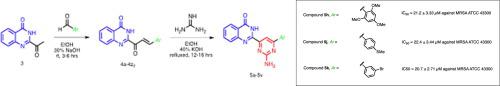
Synthesis of novel 4(3H)-quinazolinonyl aminopyrimidine derivatives has been achieved via quinazolinonyl enones which in turn were obtained from 2-acyl-4(3H)-quinazolinone. They have been assayed for biofilm inhibition against Gram-positive (methicillin-resistant Staphylococcus aureus (MRSA)) and Gram-negative bacteria (Acinetobacter baumannii). The analogues with 2,4,6-trimethoxy phenyl, 4-methylthio phenyl, and 3-bromo phenyl substituents (5h, 5j & 5k) have been shown to inhibit biofilm formation efficiently in MRSA with IC50 values of 20.7–22.4 μM). The analogues 5h and 5j have demonstrated low toxicity in human cells in vitro and can be investigated further as leads.
中文翻译:

2-(2-氨基-6-芳基嘧啶-4-基)喹唑啉-4(3H)-酮的合成和生物膜抑制研究。
通过喹唑啉酮烯酮合成了新型 4(3 H )-喹唑啉酮基氨基嘧啶衍生物,而喹唑啉酮烯酮又从 2-酰基-4(3 H )-喹唑啉酮获得。已对它们进行了针对革兰氏阳性菌(耐甲氧西林金黄色葡萄球菌(MRSA))和革兰氏阴性菌(鲍曼不动杆菌)的生物膜抑制试验。具有 2,4,6-三甲氧基苯基、4-甲硫基苯基和 3-溴苯基取代基的类似物(5h、5j和5k)已被证明可以有效抑制 MRSA 中的生物膜形成,IC 50值为 20.7–22.4 μM) 。类似物5h和5j在体外人体细胞中显示出低毒性,可以作为先导化合物进行进一步研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号