当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Decoding the intricate network of molecular interactions of a hyperstable engineered biocatalyst
Chemical Science ( IF 7.6 ) Pub Date : 2020-09-11 , DOI: 10.1039/d0sc03367g Klara Markova 1, 2 , Klaudia Chmelova 1, 2 , Sérgio M Marques 1, 2 , Philippe Carpentier 3, 4 , David Bednar 1, 2 , Jiri Damborsky 1, 2 , Martin Marek 1, 2
Chemical Science ( IF 7.6 ) Pub Date : 2020-09-11 , DOI: 10.1039/d0sc03367g Klara Markova 1, 2 , Klaudia Chmelova 1, 2 , Sérgio M Marques 1, 2 , Philippe Carpentier 3, 4 , David Bednar 1, 2 , Jiri Damborsky 1, 2 , Martin Marek 1, 2
Affiliation
|
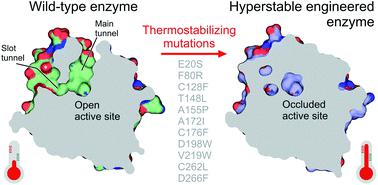
Computational design of protein catalysts with enhanced stabilities for use in research and enzyme technologies is a challenging task. Using force-field calculations and phylogenetic analysis, we previously designed the haloalkane dehalogenase DhaA115 which contains 11 mutations that confer upon it outstanding thermostability (Tm = 73.5 °C; ΔTm > 23 °C). An understanding of the structural basis of this hyperstabilization is required in order to develop computer algorithms and predictive tools. Here, we report X-ray structures of DhaA115 at 1.55 Å and 1.6 Å resolutions and their molecular dynamics trajectories, which unravel the intricate network of interactions that reinforce the αβα-sandwich architecture. Unexpectedly, mutations toward bulky aromatic amino acids at the protein surface triggered long-distance (∼27 Å) backbone changes due to cooperative effects. These cooperative interactions produced an unprecedented double-lock system that: (i) induced backbone changes, (ii) closed the molecular gates to the active site, (iii) reduced the volumes of the main and slot access tunnels, and (iv) occluded the active site. Despite these spatial restrictions, experimental tracing of the access tunnels using krypton derivative crystals demonstrates that transport of ligands is still effective. Our findings highlight key thermostabilization effects and provide a structural basis for designing new thermostable protein catalysts.
中文翻译:

解码超稳定工程生物催化剂分子相互作用的复杂网络
用于研究和酶技术的具有增强稳定性的蛋白质催化剂的计算设计是一项具有挑战性的任务。利用力场计算和系统发育分析,我们之前设计了卤代烷脱卤酶 DhaA115,它包含 11 个突变,赋予其出色的热稳定性(T m = 73.5 °C;Δ T m > 23 °C)。为了开发计算机算法和预测工具,需要了解这种超稳定的结构基础。在这里,我们报告了 DhaA115 分辨率为 1.55 Å 和 1.6 Å 的 X 射线结构及其分子动力学轨迹,这些轨迹揭示了强化 αβα 三明治结构的复杂相互作用网络。出乎意料的是,由于协同效应,蛋白质表面的大芳香氨基酸突变引发了长距离(~27 Å)主链变化。这些协作相互作用产生了前所未有的双锁系统,该系统:(i)引起主链变化,(ii)关闭活性位点的分子门,(iii)减少主通道和槽通道的体积,以及(iv)闭塞活性位点。尽管有这些空间限制,使用氪衍生物晶体对进入隧道的实验追踪表明配体的运输仍然有效。我们的研究结果强调了关键的热稳定效应,并为设计新型热稳定蛋白质催化剂提供了结构基础。
更新日期:2020-09-23
中文翻译:

解码超稳定工程生物催化剂分子相互作用的复杂网络
用于研究和酶技术的具有增强稳定性的蛋白质催化剂的计算设计是一项具有挑战性的任务。利用力场计算和系统发育分析,我们之前设计了卤代烷脱卤酶 DhaA115,它包含 11 个突变,赋予其出色的热稳定性(T m = 73.5 °C;Δ T m > 23 °C)。为了开发计算机算法和预测工具,需要了解这种超稳定的结构基础。在这里,我们报告了 DhaA115 分辨率为 1.55 Å 和 1.6 Å 的 X 射线结构及其分子动力学轨迹,这些轨迹揭示了强化 αβα 三明治结构的复杂相互作用网络。出乎意料的是,由于协同效应,蛋白质表面的大芳香氨基酸突变引发了长距离(~27 Å)主链变化。这些协作相互作用产生了前所未有的双锁系统,该系统:(i)引起主链变化,(ii)关闭活性位点的分子门,(iii)减少主通道和槽通道的体积,以及(iv)闭塞活性位点。尽管有这些空间限制,使用氪衍生物晶体对进入隧道的实验追踪表明配体的运输仍然有效。我们的研究结果强调了关键的热稳定效应,并为设计新型热稳定蛋白质催化剂提供了结构基础。









































 京公网安备 11010802027423号
京公网安备 11010802027423号