当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Seeing luminescence appear as crystals crumble. Isolation and subsequent self-association of individual [(C6H11NC)2Au]+ ions in crystals
Chemical Science ( IF 7.6 ) Pub Date : 2020-09-10 , DOI: 10.1039/d0sc03299a Lucy M C Luong 1 , Christopher D Lowe 1 , Alexandria V Adams 1 , Venoos Moshayedi 1 , Marilyn M Olmstead 1 , Alan L Balch 1
Chemical Science ( IF 7.6 ) Pub Date : 2020-09-10 , DOI: 10.1039/d0sc03299a Lucy M C Luong 1 , Christopher D Lowe 1 , Alexandria V Adams 1 , Venoos Moshayedi 1 , Marilyn M Olmstead 1 , Alan L Balch 1
Affiliation
|
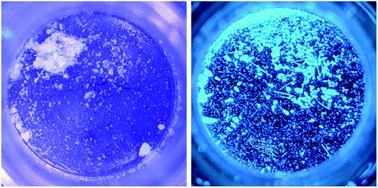
Non-luminescent, isostructural crystals of [(C6H11NC)2Au](EF6)·C6H6 (E = As, Sb) lose benzene upon standing in air to produce green luminescent (E = As) or blue luminescent (E = Sb) powders. Previous studies have shown that the two-coordinate cation, [(C6H11NC)2Au]+, self-associates to form luminescent crystals that contain linear or nearly linear chains of cations and display unusual polymorphic, vapochromic, and/or thermochromic properties. Here, we report the formation of non-luminescent crystalline salts in which individual [(C6H11NC)2Au]+ ions are isolated from one another. In [(C6H11NC)2Au](BArF24) ((BArF24)− is tetrakis[3,5-bis(trifluoromethyl)phenyl]borate) each cation is surrounded by two anions that prohibit any close approach of the gold ions. Crystallization of [(C6H11NC)2Au](EF6) (E = As or Sb, but not P) from benzene solution produces colorless, non-emissive crystals of the solvates [(C6H11NC)2Au](EF6)·C6H6. These two solvates are isostructural and contain columns in which cations and benzene molecules alternate. With the benzene molecules separating the cations, the shortest distances between gold ions are 6.936(2) Å for E = As and 6.9717(19) Å for E = Sb. Upon removal from the mother liquor, these crystals crack due to the loss of benzene from the crystal and form luminescent powders. Crystals of [(C6H11NC)2Au](SbF6)·C6H6 that powder out form a pale yellow powder with a blue luminescence with emission spectra and powder X-ray diffraction data that show that the previously characterized [(C6H11NC)2Au](SbF6) is formed. In the process, the distances between the gold(I) ions decrease to ∼3 Å and half of the cyclohexyl groups move from an axial orientation to an equatorial one. Remarkably, when crystals of [(C6H11NC)2Au](AsF6)·C6H6 stand in air, they lose benzene and are converted into the yellow, green-luminescent polymorph of [(C6H11NC)2Au](AsF6) rather than the colorless, blue-luminescent polymorph. Paradoxically, the yellow, green-luminescent powder that forms as well as authentic crystals of the yellow, green-luminescent polymorph of [(C6H11NC)2Au](AsF6) are sensitive to benzene vapor and are converted by exposure to benzene vapor into the colorless, blue-luminescent polymorph.
中文翻译:

看到晶体破碎时出现发光。晶体中单个 [(C6H11NC)2Au]+ 离子的分离和随后的自缔合
[(C 6 H 11 NC) 2 Au](EF 6 )·C 6 H 6 (E = As, Sb)的非发光同构晶体在空气中放置时失去苯,产生绿色发光 (E = As) 或蓝色发光(E = Sb)粉末。先前的研究表明,二配位阳离子 [(C 6 H 11 NC) 2 Au] +自缔合形成包含线性或近线性阳离子链的发光晶体,并显示出不寻常的多晶型、气色和/或热致变色特性。在这里,我们报道了不发光晶体盐的形成,其中各个[(C 6 H 11 NC) 2 Au] +离子彼此分离。在[(C 6 H 11 NC) 2 Au](BArF 24 ) ((BArF 24 ) −是四[3,5-双(三氟甲基)苯基]硼酸盐)中,每个阳离子被两个阴离子包围,这阻止了任何接近的阴离子金离子。[(C 6 H 11 NC) 2 Au](EF 6 )(E = As 或 Sb,但不是 P)从苯溶液中结晶,产生无色、非发射性溶剂化物晶体 [(C 6 H 11 NC) 2 Au](EF 6 )·C 6 H 6。这两种溶剂化物是同构的,并含有阳离子和苯分子交替的柱。由于苯分子分隔阳离子,金离子之间的最短距离为 6.936(2) Å(E = As)和 6.9717(19) Å(E = Sb)。从母液中取出后,这些晶体由于晶体中苯的损失而破裂并形成发光粉末。粉末化的[(C 6 H 11 NC) 2 Au](SbF 6 )·C 6 H 6晶体形成具有蓝色发光的浅黄色粉末,发射光谱和粉末X射线衍射数据表明先前表征的形成[(C 6 H 11 NC) 2 Au](SbF 6 )。在此过程中,金( I )离子之间的距离减小至~3 Å,并且一半的环己基从轴向方向移动到赤道方向。值得注意的是,当[(C 6 H 11 NC) 2 Au](AsF 6 )·C 6 H 6晶体放置在空气中,它们失去苯并转化为[(C 6 H 11 NC) 2 Au](AsF 6 )的黄色、绿色发光多晶型物,而不是无色、蓝色发光多晶型物。矛盾的是,形成的黄色、绿色发光粉末以及[(C 6 H 11 NC) 2 Au](AsF 6 )的黄色、绿色发光多晶型物的真实晶体对苯蒸气敏感,并且通过暴露而转化将苯蒸气转化为无色、蓝色发光的多晶型物。
更新日期:2020-09-23
中文翻译:

看到晶体破碎时出现发光。晶体中单个 [(C6H11NC)2Au]+ 离子的分离和随后的自缔合
[(C 6 H 11 NC) 2 Au](EF 6 )·C 6 H 6 (E = As, Sb)的非发光同构晶体在空气中放置时失去苯,产生绿色发光 (E = As) 或蓝色发光(E = Sb)粉末。先前的研究表明,二配位阳离子 [(C 6 H 11 NC) 2 Au] +自缔合形成包含线性或近线性阳离子链的发光晶体,并显示出不寻常的多晶型、气色和/或热致变色特性。在这里,我们报道了不发光晶体盐的形成,其中各个[(C 6 H 11 NC) 2 Au] +离子彼此分离。在[(C 6 H 11 NC) 2 Au](BArF 24 ) ((BArF 24 ) −是四[3,5-双(三氟甲基)苯基]硼酸盐)中,每个阳离子被两个阴离子包围,这阻止了任何接近的阴离子金离子。[(C 6 H 11 NC) 2 Au](EF 6 )(E = As 或 Sb,但不是 P)从苯溶液中结晶,产生无色、非发射性溶剂化物晶体 [(C 6 H 11 NC) 2 Au](EF 6 )·C 6 H 6。这两种溶剂化物是同构的,并含有阳离子和苯分子交替的柱。由于苯分子分隔阳离子,金离子之间的最短距离为 6.936(2) Å(E = As)和 6.9717(19) Å(E = Sb)。从母液中取出后,这些晶体由于晶体中苯的损失而破裂并形成发光粉末。粉末化的[(C 6 H 11 NC) 2 Au](SbF 6 )·C 6 H 6晶体形成具有蓝色发光的浅黄色粉末,发射光谱和粉末X射线衍射数据表明先前表征的形成[(C 6 H 11 NC) 2 Au](SbF 6 )。在此过程中,金( I )离子之间的距离减小至~3 Å,并且一半的环己基从轴向方向移动到赤道方向。值得注意的是,当[(C 6 H 11 NC) 2 Au](AsF 6 )·C 6 H 6晶体放置在空气中,它们失去苯并转化为[(C 6 H 11 NC) 2 Au](AsF 6 )的黄色、绿色发光多晶型物,而不是无色、蓝色发光多晶型物。矛盾的是,形成的黄色、绿色发光粉末以及[(C 6 H 11 NC) 2 Au](AsF 6 )的黄色、绿色发光多晶型物的真实晶体对苯蒸气敏感,并且通过暴露而转化将苯蒸气转化为无色、蓝色发光的多晶型物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号