当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Syntheses of SGLT2 Inhibitors by Ni- and Pd-Catalyzed Fukuyama Coupling Reactions.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-09-10 , DOI: 10.1021/acs.joc.0c01635 Jalindar Talode 1 , Daiki Kato 1 , Haruki Nagae 1 , Hayato Tsurugi 1 , Masahiko Seki 2 , Kazushi Mashima 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-09-10 , DOI: 10.1021/acs.joc.0c01635 Jalindar Talode 1 , Daiki Kato 1 , Haruki Nagae 1 , Hayato Tsurugi 1 , Masahiko Seki 2 , Kazushi Mashima 1
Affiliation
|
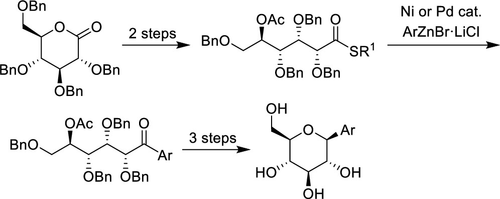
Nickel- and palladium-catalyzed Fukuyama coupling reactions of a d-gluconolactone-derived thioester with arylzinc reagents at ambient temperature provided the corresponding multifunctional aryl ketones in high yield. Ligand screening for the nickel-catalyzed Fukuyama coupling reactions indicated that 1,2-bis(dicyclohexylphosphino)ethane (dCype) served as a superior supporting ligand to improve the product yield. In addition, Pd/C was a practical alternative that enabled ligand-free Fukuyama coupling reactions and was efficiently applied to the key C–C bond-forming step to prepare canagliflozin and dapagliflozin, which are diabetic SGLT2 inhibitors of current interest.
中文翻译:

Ni和Pd催化的福山偶联反应合成SGLT2抑制剂。
d-葡糖酸内酯衍生的硫酯与芳基锌试剂的镍和钯催化的Fukuyama偶联反应在室温下以高收率提供了相应的多功能芳基酮。镍催化的福山偶联反应的配体筛选表明,1,2-双(二环己基膦基)乙烷(dCype)可作为优良的支持配体来提高产品收率。此外,Pd / C是一种可行的替代方法,可实现无配体的福山偶联反应,并有效地应用于关键的C–C键形成步骤,以制备canagliflozin和dapagliflozin,它们是目前关注的糖尿病SGLT2抑制剂。
更新日期:2020-10-02
中文翻译:

Ni和Pd催化的福山偶联反应合成SGLT2抑制剂。
d-葡糖酸内酯衍生的硫酯与芳基锌试剂的镍和钯催化的Fukuyama偶联反应在室温下以高收率提供了相应的多功能芳基酮。镍催化的福山偶联反应的配体筛选表明,1,2-双(二环己基膦基)乙烷(dCype)可作为优良的支持配体来提高产品收率。此外,Pd / C是一种可行的替代方法,可实现无配体的福山偶联反应,并有效地应用于关键的C–C键形成步骤,以制备canagliflozin和dapagliflozin,它们是目前关注的糖尿病SGLT2抑制剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号