当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic Origin of Photoredox Catalysis Involving Iron(II) Polypyridyl Chromophores
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-09-11 , DOI: 10.1021/jacs.0c08389 Matthew D Woodhouse 1 , James K McCusker 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-09-11 , DOI: 10.1021/jacs.0c08389 Matthew D Woodhouse 1 , James K McCusker 1
Affiliation
|
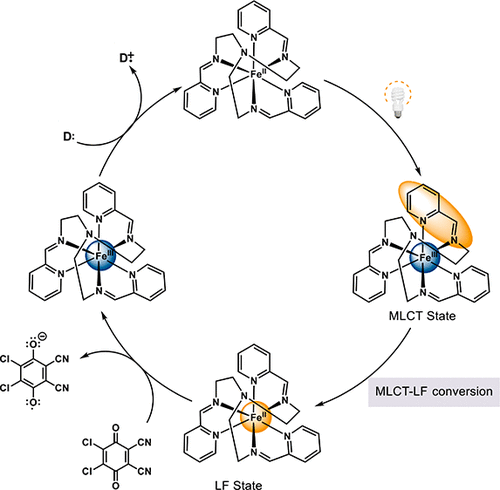
Photoredox catalysis employing ruthenium- and iridium-based chromophores have been the subject of considerable research. However, the natural abundance of these elements are among the lowest on the periodic table, a fact that has led to an interest in developing chromophores based on earth-abundant transition metals that can perform the same function. There have been reports of using FeII-based polypyridyl complexes as photocatalysts, but there is limited mechanistic information pertaining to the nature of their reactivity in the context of photoredox chemistry. Herein, we report the results of bimolecular quenching studies between [Fe(tren(py)3)]2+ (where tren(py)3 = tris(2-pyridyl-methylimino-ethyl)amine) and a series of benzoquinoid acceptors. The data provide direct evidence of electron transfer involving the lowest-energy ligand-field excited state of the Fe(II)-based photosensitizer, definitively establishing that Fe(II) polypyridyl complexes can engage in photoinduced redox reactions but by a mechanism that is fundamentally different than the MLCT-based chemistry endemic to their second- and third-row congeners.
中文翻译:

涉及铁(II)多吡啶基发色团的光氧化还原催化的机理起源
使用基于钌和铱的发色团的光氧化还原催化已成为大量研究的主题。然而,这些元素的自然丰度在元素周期表中是最低的,这一事实导致人们对开发基于地球上丰富的过渡金属的发色团产生了兴趣,这些发色团可以执行相同的功能。有使用基于 FeII 的多吡啶基配合物作为光催化剂的报道,但关于它们在光氧化还原化学背景下的反应性质的机制信息有限。在此,我们报告了 [Fe(tren(py)3)]2+(其中 tren(py)3 = 三(2-吡啶基-甲基亚氨基-乙基)胺)和一系列苯并醌受体之间的双分子淬灭研究结果。
更新日期:2020-09-11
中文翻译:

涉及铁(II)多吡啶基发色团的光氧化还原催化的机理起源
使用基于钌和铱的发色团的光氧化还原催化已成为大量研究的主题。然而,这些元素的自然丰度在元素周期表中是最低的,这一事实导致人们对开发基于地球上丰富的过渡金属的发色团产生了兴趣,这些发色团可以执行相同的功能。有使用基于 FeII 的多吡啶基配合物作为光催化剂的报道,但关于它们在光氧化还原化学背景下的反应性质的机制信息有限。在此,我们报告了 [Fe(tren(py)3)]2+(其中 tren(py)3 = 三(2-吡啶基-甲基亚氨基-乙基)胺)和一系列苯并醌受体之间的双分子淬灭研究结果。



























 京公网安备 11010802027423号
京公网安备 11010802027423号