当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient Synthesis of Cannabigerol, Grifolin, and Piperogalin via Alumina-Promoted Allylation
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-09-10 , DOI: 10.1021/acs.jnatprod.0c00131 Nicholas G Jentsch 1 , Xiong Zhang 1 , Jakob Magolan 1
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-09-10 , DOI: 10.1021/acs.jnatprod.0c00131 Nicholas G Jentsch 1 , Xiong Zhang 1 , Jakob Magolan 1
Affiliation
|
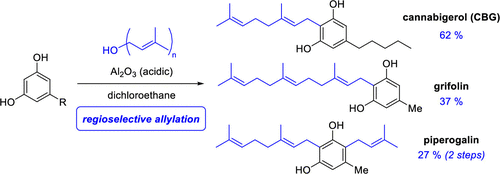
The synthesis of three phenolic natural products has been accomplished with unprecedented efficiency using a new alumina-promoted regioselective aromatic allylation reaction. Cannabigerol and grifolin were prepared in one step from the inexpensive 5-alkyl-resorcinols olivetol and orcinol. Piperogalin was synthesized, for the first time, via two sequential allylations of orcinol with geraniol and prenol.
中文翻译:

通过氧化铝促进的烯丙基化有效合成大麻酚、格里福林和哌醋甲酯
使用新的氧化铝促进的区域选择性芳香烯丙基化反应以前所未有的效率完成了三种酚类天然产物的合成。Cannabigerol 和 grifolin 是从廉价的 5-烷基间苯二酚 Olivetol 和 orcinol 一步制备的。首次通过地黄酚与香叶醇和异戊二烯醇的两次连续烯丙基化合成了哌醋甲酯。
更新日期:2020-09-25
中文翻译:

通过氧化铝促进的烯丙基化有效合成大麻酚、格里福林和哌醋甲酯
使用新的氧化铝促进的区域选择性芳香烯丙基化反应以前所未有的效率完成了三种酚类天然产物的合成。Cannabigerol 和 grifolin 是从廉价的 5-烷基间苯二酚 Olivetol 和 orcinol 一步制备的。首次通过地黄酚与香叶醇和异戊二烯醇的两次连续烯丙基化合成了哌醋甲酯。











































 京公网安备 11010802027423号
京公网安备 11010802027423号