当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of PF-06835919: A Potent Inhibitor of Ketohexokinase (KHK) for the Treatment of Metabolic Disorders Driven by the Overconsumption of Fructose.
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-09-10 , DOI: 10.1021/acs.jmedchem.0c00944 Kentaro Futatsugi 1 , Aaron C Smith 2 , Meihua Tu 1 , Brian Raymer 1 , Kay Ahn 3 , Steven B Coffey 2 , Matthew S Dowling 2 , Dilinie P Fernando 2 , Jemy A Gutierrez 3 , Kim Huard 1 , Jayasankar Jasti 2 , Amit S Kalgutkar 1 , John D Knafels 2 , Jayvardhan Pandit 2 , Kevin D Parris 2 , Sylvie Perez 3 , Jeffrey A Pfefferkorn 3 , David A Price 1 , Tim Ryder 2 , Andre Shavnya 2 , Ingrid A Stock 2 , Andy S Tsai 2 , Gregory J Tesz 3 , Benjamin A Thuma 2 , Yan Weng 1 , Hanna M Wisniewska 2 , Gang Xing 3 , Jun Zhou 4 , Thomas V Magee 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-09-10 , DOI: 10.1021/acs.jmedchem.0c00944 Kentaro Futatsugi 1 , Aaron C Smith 2 , Meihua Tu 1 , Brian Raymer 1 , Kay Ahn 3 , Steven B Coffey 2 , Matthew S Dowling 2 , Dilinie P Fernando 2 , Jemy A Gutierrez 3 , Kim Huard 1 , Jayasankar Jasti 2 , Amit S Kalgutkar 1 , John D Knafels 2 , Jayvardhan Pandit 2 , Kevin D Parris 2 , Sylvie Perez 3 , Jeffrey A Pfefferkorn 3 , David A Price 1 , Tim Ryder 2 , Andre Shavnya 2 , Ingrid A Stock 2 , Andy S Tsai 2 , Gregory J Tesz 3 , Benjamin A Thuma 2 , Yan Weng 1 , Hanna M Wisniewska 2 , Gang Xing 3 , Jun Zhou 4 , Thomas V Magee 1
Affiliation
|
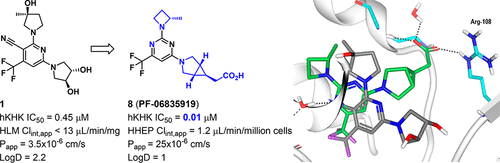
Increased fructose consumption and its subsequent metabolism have been implicated in metabolic disorders such as nonalcoholic fatty liver disease and steatohepatitis (NAFLD/NASH) and insulin resistance. Ketohexokinase (KHK) converts fructose to fructose-1-phosphate (F1P) in the first step of the metabolic cascade. Herein we report the discovery of a first-in-class KHK inhibitor, PF-06835919 (8), currently in phase 2 clinical trials. The discovery of 8 was built upon our originally reported, fragment-derived lead 1 and the recognition of an alternative, rotated binding mode upon changing the ribose-pocket binding moiety from a pyrrolidinyl to an azetidinyl ring system. This new binding mode enabled efficient exploration of the vector directed at the Arg-108 residue, leading to the identification of highly potent 3-azabicyclo[3.1.0]hexane acetic acid-based KHK inhibitors by combined use of parallel medicinal chemistry and structure-based drug design.
中文翻译:

PF-06835919的发现:酮己糖激酶(KHK)的强效抑制剂,用于治疗因果糖过量消费引起的代谢紊乱。
果糖的摄入增加及其随后的代谢与非酒精性脂肪肝,脂肪性肝炎(NAFLD / NASH)和胰岛素抵抗等代谢异常有关。在代谢级联反应的第一步中,酮己糖激酶(KHK)将果糖转化为果糖-1-磷酸(F1P)。本文中,我们报告了目前处于第2期临床试验中的一流KHK抑制剂PF-06835919(8)的发现。8的发现建立在我们最初报道的片段衍生的铅1的基础上当核糖口袋结合部分从吡咯烷基变为氮杂环丁烷基环系统时,就认识到了另一种旋转结合方式。这种新的结合模式使得能够有效探索针对Arg-108残基的载体,从而通过平行药物化学和结构联用的方法鉴定出了高效的基于3-氮杂双环[3.1.0]己烷乙酸的KHK抑制剂。基于药物的设计。
更新日期:2020-09-10
中文翻译:

PF-06835919的发现:酮己糖激酶(KHK)的强效抑制剂,用于治疗因果糖过量消费引起的代谢紊乱。
果糖的摄入增加及其随后的代谢与非酒精性脂肪肝,脂肪性肝炎(NAFLD / NASH)和胰岛素抵抗等代谢异常有关。在代谢级联反应的第一步中,酮己糖激酶(KHK)将果糖转化为果糖-1-磷酸(F1P)。本文中,我们报告了目前处于第2期临床试验中的一流KHK抑制剂PF-06835919(8)的发现。8的发现建立在我们最初报道的片段衍生的铅1的基础上当核糖口袋结合部分从吡咯烷基变为氮杂环丁烷基环系统时,就认识到了另一种旋转结合方式。这种新的结合模式使得能够有效探索针对Arg-108残基的载体,从而通过平行药物化学和结构联用的方法鉴定出了高效的基于3-氮杂双环[3.1.0]己烷乙酸的KHK抑制剂。基于药物的设计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号