当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Isobaric Vapor–Liquid Equilibrium Measurements and Modeling of Water + Monoethylene Glycol + NaCl Mixtures
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-09-10 , DOI: 10.1021/acs.jced.0c00351 Mario H. Moura-Neto 1 , Mateus F. Monteiro 1 , André L. N. Mota 2 , Dannielle J. Silva 1 , Jailton F. do Nascimento 3 , Leonardo S. Pereira 3 , Osvaldo Chiavone-Filho 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-09-10 , DOI: 10.1021/acs.jced.0c00351 Mario H. Moura-Neto 1 , Mateus F. Monteiro 1 , André L. N. Mota 2 , Dannielle J. Silva 1 , Jailton F. do Nascimento 3 , Leonardo S. Pereira 3 , Osvaldo Chiavone-Filho 1
Affiliation
|
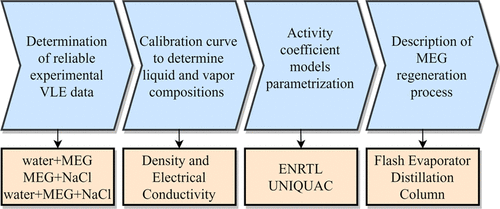
Vapor–liquid equilibrium (VLE) data for aqueous systems in the presence of electrolytes have many industrial applications. VLE data for water + monoethylene glycol (MEG) mixtures in the presence of sodium chloride at low pressures are important to describe the effects of composition, temperature, and pressure on a MEG (gas hydrate inhibitor) regeneration unit. A modified version of the Othmer ebulliometer was applied to measure reliable VLE data for water + MEG + NaCl at 101.325, 65, and 35 kPa. Binary systems (water + MEG and MEG + NaCl) were also experimentally studied. Vapor pressures for water and MEG were determined and compared with the selected literature data via Antoine correlation. The electrolyte nonrandom two-liquid and universal quasi-chemical activity coefficient models were successfully parameterized to describe the VLE behavior for water + MEG + NaCl systems. Thermodynamic consistence of the data sets was also checked. Interaction parameter estimation followed a systematic strategy: (1) water−MEG, (2) water−NaCl, and finally (3) MEG−NaCl with the experimental data of MEG + NaCl and ternary data. MEG + NaCl solutions presented an inverted colligative property, that is, the addition of salt decreases the boiling point. VLE data indicated that water separation is less efficient due to the addition of salt. The parameterized models allow an evaluation of the MEG regeneration process as a function of temperature, pressure, and composition.
中文翻译:

水+单乙二醇+ NaCl混合物的等压蒸气-液体平衡测量和建模
存在电解质的水系统的汽液平衡(VLE)数据具有许多工业应用。在低压下存在氯化钠的情况下,水+单乙二醇(MEG)混合物的VLE数据对于描述组成,温度和压力对MEG(气体水合物抑制剂)再生装置的影响非常重要。使用Othmer浊度仪的改进版来测量水+ MEG + NaCl在101.325、65和35 kPa时的可靠VLE数据。还通过实验研究了二元体系(水+ MEG和MEG + NaCl)。确定水和MEG的蒸气压,并通过Antoine相关性将其与所选文献数据进行比较。电解质非随机两液和通用准化学活性系数模型已成功参数化,以描述水+ MEG + NaCl系统的VLE行为。还检查了数据集的热力学一致性。相互作用参数的估计遵循一种系统策略:(1)水-MEG,(2)水-NaCl,最后(3)MEG-NaCl,其中包含MEG + NaCl的实验数据和三元数据。MEG + NaCl溶液表现出倒数的依数性质,即添加盐会降低沸点。VLE数据表明,由于添加了盐,水分离效率较低。参数化的模型允许根据温度,压力和组成对MEG再生过程进行评估。还检查了数据集的热力学一致性。相互作用参数的估计遵循一种系统策略:(1)水-MEG,(2)水-NaCl,最后(3)MEG-NaCl,其中包含MEG + NaCl的实验数据和三元数据。MEG + NaCl溶液表现出倒数的依数性质,即添加盐会降低沸点。VLE数据表明,由于添加了盐,水分离效率较低。参数化的模型允许根据温度,压力和组成对MEG再生过程进行评估。还检查了数据集的热力学一致性。相互作用参数的估计遵循一种系统策略:(1)水-MEG,(2)水-NaCl,最后(3)MEG-NaCl,其中包含MEG + NaCl的实验数据和三元数据。MEG + NaCl溶液表现出倒数的依数性质,即添加盐会降低沸点。VLE数据表明,由于添加了盐,水分离效率较低。参数化的模型允许根据温度,压力和组成对MEG再生过程进行评估。盐的添加降低了沸点。VLE数据表明,由于添加了盐,水分离效率较低。参数化的模型允许根据温度,压力和组成对MEG再生过程进行评估。盐的添加降低了沸点。VLE数据表明,由于添加了盐,水分离效率较低。参数化的模型允许根据温度,压力和组成对MEG再生过程进行评估。
更新日期:2020-10-08
中文翻译:

水+单乙二醇+ NaCl混合物的等压蒸气-液体平衡测量和建模
存在电解质的水系统的汽液平衡(VLE)数据具有许多工业应用。在低压下存在氯化钠的情况下,水+单乙二醇(MEG)混合物的VLE数据对于描述组成,温度和压力对MEG(气体水合物抑制剂)再生装置的影响非常重要。使用Othmer浊度仪的改进版来测量水+ MEG + NaCl在101.325、65和35 kPa时的可靠VLE数据。还通过实验研究了二元体系(水+ MEG和MEG + NaCl)。确定水和MEG的蒸气压,并通过Antoine相关性将其与所选文献数据进行比较。电解质非随机两液和通用准化学活性系数模型已成功参数化,以描述水+ MEG + NaCl系统的VLE行为。还检查了数据集的热力学一致性。相互作用参数的估计遵循一种系统策略:(1)水-MEG,(2)水-NaCl,最后(3)MEG-NaCl,其中包含MEG + NaCl的实验数据和三元数据。MEG + NaCl溶液表现出倒数的依数性质,即添加盐会降低沸点。VLE数据表明,由于添加了盐,水分离效率较低。参数化的模型允许根据温度,压力和组成对MEG再生过程进行评估。还检查了数据集的热力学一致性。相互作用参数的估计遵循一种系统策略:(1)水-MEG,(2)水-NaCl,最后(3)MEG-NaCl,其中包含MEG + NaCl的实验数据和三元数据。MEG + NaCl溶液表现出倒数的依数性质,即添加盐会降低沸点。VLE数据表明,由于添加了盐,水分离效率较低。参数化的模型允许根据温度,压力和组成对MEG再生过程进行评估。还检查了数据集的热力学一致性。相互作用参数的估计遵循一种系统策略:(1)水-MEG,(2)水-NaCl,最后(3)MEG-NaCl,其中包含MEG + NaCl的实验数据和三元数据。MEG + NaCl溶液表现出倒数的依数性质,即添加盐会降低沸点。VLE数据表明,由于添加了盐,水分离效率较低。参数化的模型允许根据温度,压力和组成对MEG再生过程进行评估。盐的添加降低了沸点。VLE数据表明,由于添加了盐,水分离效率较低。参数化的模型允许根据温度,压力和组成对MEG再生过程进行评估。盐的添加降低了沸点。VLE数据表明,由于添加了盐,水分离效率较低。参数化的模型允许根据温度,压力和组成对MEG再生过程进行评估。











































 京公网安备 11010802027423号
京公网安备 11010802027423号