当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetic Modeling of Conversion of Levulinic Acid to Valeric Acid in Supercritical Water Using the Density Functional Theory Framework
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2020-09-11 , DOI: 10.1021/acs.iecr.0c02807 Pritam Chakraborty 1 , Kushagra Agrawal 1 , Nanda Kishore 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2020-09-11 , DOI: 10.1021/acs.iecr.0c02807 Pritam Chakraborty 1 , Kushagra Agrawal 1 , Nanda Kishore 1
Affiliation
|
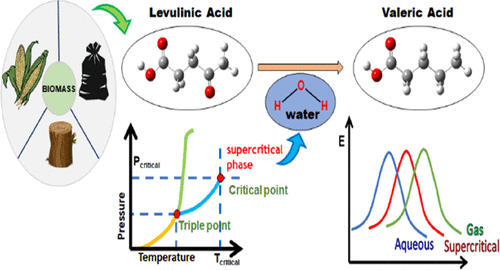
The faster depleting natural reserves of fossil fuel and growing global climate change crisis have shifted the focus of researchers toward the extraction of bio-fuel and value-added chemicals from biomass. In this quest, supercritical (SC) water as a medium has been experimentally explored to derive bio-oil from biomass and deoxygenate the oxygenated compounds of it. Levulinic acid (LA) and pentanoic acid or valeric acid (VA) are two standard value-added products obtained from the biomass treatment. Thus, in this study, the authors report the kinetics of the conversion of levulinic acid to valeric acid at four different supercritical conditions using an implicit solvation model available within the framework of density functional theory (DFT) and compare them with their gas and aqueous phase counterparts. Prior to obtaining the new results, the present approach is first benchmarked with the existing experimental and theoretical literature under the supercritical water conditions. The conversion of levulinic acid is studied in two competing pathways. For each of the reaction pathways, the enthalpy and Gibbs free energy changes have been discussed. It is found that the production of valeric acid is equally likely to proceed by the protonation of the fourth carbon of the acid or by the protonation of the oxo-group at the fourth carbon atom. The solvent effects are found to be favorable, especially under two supercritical conditions, SC1 (ρ = 0.089 g/cc, T = 773 K, P = 250 bar) and SC2 (ρ = 0.109 g/cc, T = 723 K, P = 250 bar) compared to SC3 (ρ = 0.190 g/cc, T = 700 K, P = 304 bar) and SC4 (ρ = 0.360 g/cc, T = 723 K, P = 463 bar) conditions.
中文翻译:

密度泛函理论框架的超临界水中白蛋白转化为戊酸的动力学模型
化石燃料自然储备的快速消耗和全球气候变化危机的加剧,已将研究人员的重点转向从生物质中提取生物燃料和增值化学品。在此探索中,已对以超临界(SC)水为介质的实验进行了探索,以从生物质中提取生物油并将其含氧化合物脱氧。左旋维甲酸(LA)和戊酸或戊酸(VA)是从生物质处理中获得的两个标准增值产品。因此,在这项研究中,作者使用在密度泛函理论(DFT)框架内可用的隐式溶剂化模型,报告了在四种不同超临界条件下乙酰丙酸向戊酸转化的动力学,并将其与气相和水相进行了比较。同行。在获得新结果之前,该方法首先在超临界水条件下以现有的实验和理论文献为基准。在两个竞争途径中研究了乙酰丙酸的转化。对于每种反应途径,已经讨论了焓和吉布斯自由能的变化。发现戊酸的生产同样可能通过酸的第四碳的质子化或通过第四碳原子上的氧代基的质子化来进行。发现溶剂效应是有利的,尤其是在两个超临界条件SC1(ρ= 0.089 g / cc,对于每种反应途径,已经讨论了焓和吉布斯自由能的变化。发现戊酸的生产同样可能通过酸的第四碳的质子化或通过第四碳原子上的氧代基的质子化来进行。发现溶剂效应是有利的,尤其是在两个超临界条件SC1(ρ= 0.089 g / cc,对于每种反应途径,已经讨论了焓和吉布斯自由能的变化。发现戊酸的生产同样可能通过酸的第四碳的质子化或通过第四碳原子上的氧代基的质子化来进行。发现溶剂效应是有利的,尤其是在两个超临界条件SC1(ρ= 0.089 g / cc,T = 773 K,P = 250 bar)和SC2(ρ= 0.109 g / cc,T = 723 K,P = 250 bar),而SC3(ρ= 0.190 g / cc,T = 700 K,P = 304 bar )和SC4(ρ= 0.360 g / cc,T = 723 K,P = 463 bar)条件。
更新日期:2020-10-15
中文翻译:

密度泛函理论框架的超临界水中白蛋白转化为戊酸的动力学模型
化石燃料自然储备的快速消耗和全球气候变化危机的加剧,已将研究人员的重点转向从生物质中提取生物燃料和增值化学品。在此探索中,已对以超临界(SC)水为介质的实验进行了探索,以从生物质中提取生物油并将其含氧化合物脱氧。左旋维甲酸(LA)和戊酸或戊酸(VA)是从生物质处理中获得的两个标准增值产品。因此,在这项研究中,作者使用在密度泛函理论(DFT)框架内可用的隐式溶剂化模型,报告了在四种不同超临界条件下乙酰丙酸向戊酸转化的动力学,并将其与气相和水相进行了比较。同行。在获得新结果之前,该方法首先在超临界水条件下以现有的实验和理论文献为基准。在两个竞争途径中研究了乙酰丙酸的转化。对于每种反应途径,已经讨论了焓和吉布斯自由能的变化。发现戊酸的生产同样可能通过酸的第四碳的质子化或通过第四碳原子上的氧代基的质子化来进行。发现溶剂效应是有利的,尤其是在两个超临界条件SC1(ρ= 0.089 g / cc,对于每种反应途径,已经讨论了焓和吉布斯自由能的变化。发现戊酸的生产同样可能通过酸的第四碳的质子化或通过第四碳原子上的氧代基的质子化来进行。发现溶剂效应是有利的,尤其是在两个超临界条件SC1(ρ= 0.089 g / cc,对于每种反应途径,已经讨论了焓和吉布斯自由能的变化。发现戊酸的生产同样可能通过酸的第四碳的质子化或通过第四碳原子上的氧代基的质子化来进行。发现溶剂效应是有利的,尤其是在两个超临界条件SC1(ρ= 0.089 g / cc,T = 773 K,P = 250 bar)和SC2(ρ= 0.109 g / cc,T = 723 K,P = 250 bar),而SC3(ρ= 0.190 g / cc,T = 700 K,P = 304 bar )和SC4(ρ= 0.360 g / cc,T = 723 K,P = 463 bar)条件。











































 京公网安备 11010802027423号
京公网安备 11010802027423号