当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Hydrodehalogenation of Haloacetic Acids: A Kinetic Study
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2020-09-11 , DOI: 10.1021/acs.iecr.0c03495 Julia Nieto-Sandoval 1 , Esther Gomez-Herrero 1 , Ferdaus El Morabet 1 , Macarena Munoz 1 , Zahara M. de Pedro 1 , Jose A. Casas 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2020-09-11 , DOI: 10.1021/acs.iecr.0c03495 Julia Nieto-Sandoval 1 , Esther Gomez-Herrero 1 , Ferdaus El Morabet 1 , Macarena Munoz 1 , Zahara M. de Pedro 1 , Jose A. Casas 1
Affiliation
|
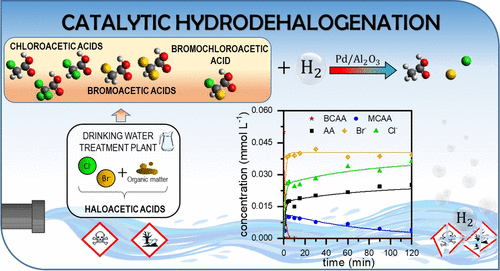
Haloacetic acids (HAAs) are undesired halogenated byproducts commonly generated upon oxidation treatments carried out in drinking water treatment plants. In this work, the removal of a representative group of these hazardous species (monochloroacetic acid, dichloroacetic acid, trichloroacetic acid, monobromoacetic acid, dibromoacetic acid, and bromochloroacetic acid) by catalytic hydrodehalogenation (HDH) was investigated and kinetic models were accordingly developed. Complete dehalogenation of all these pollutants (0.05 mmol L–1) was achieved using a Pd/Al2O3 catalyst (0.5 g L–1), although it was found that their reactivity depended on the nature and number of halogen substituents present in the HAA molecule. In general, bromoacetic acids showed higher reactivity than the chlorinated ones, which was attributed to the lower C-X bond dissociation energy. On the other hand, while the chloroacetic acid reactivity increased with the chlorination degree of the molecule, particularly from one to two Cl substituents, the bromoacetic acids did not show higher reaction rates with the increase in Br substituents. Based on the results obtained, different reaction pathways, via stepwise and/or concerted reactions, were proposed for the HDH of the HAAs. Consistent with those pathways, kinetic models were also developed, which allowed describing successfully the experimental data.
中文翻译:

卤代乙酸的催化加氢脱卤:动力学研究
卤乙酸(HAA)是在饮用水处理厂中进行氧化处理后通常产生的不希望的卤化副产物。在这项工作中,研究了通过催化加氢脱卤(HDH)去除这些危险物种(一氯乙酸,二氯乙酸,三氯乙酸,一溴乙酸,二溴乙酸和溴氯乙酸)的代表性组,并据此开发了动力学模型。使用Pd / Al 2 O 3催化剂(0.5 g L –1)可实现所有这些污染物(0.05 mmol L –1)的完全脱卤。),尽管发现它们的反应性取决于HAA分子中存在的卤素取代基的性质和数量。通常,溴乙酸显示出比氯代乙酸更高的反应性,这归因于较低的CX键解离能。另一方面,尽管氯乙酸反应性随分子的氯化度而增加,特别是从一个到两个Cl取代基,但随着Br取代基的增加,溴乙酸没有显示出更高的反应速率。基于获得的结果,提出了通过逐步和/或协同反应的不同反应途径用于HAA的HDH。与这些途径相一致,还开发了动力学模型,可以成功地描述实验数据。
更新日期:2020-10-07
中文翻译:

卤代乙酸的催化加氢脱卤:动力学研究
卤乙酸(HAA)是在饮用水处理厂中进行氧化处理后通常产生的不希望的卤化副产物。在这项工作中,研究了通过催化加氢脱卤(HDH)去除这些危险物种(一氯乙酸,二氯乙酸,三氯乙酸,一溴乙酸,二溴乙酸和溴氯乙酸)的代表性组,并据此开发了动力学模型。使用Pd / Al 2 O 3催化剂(0.5 g L –1)可实现所有这些污染物(0.05 mmol L –1)的完全脱卤。),尽管发现它们的反应性取决于HAA分子中存在的卤素取代基的性质和数量。通常,溴乙酸显示出比氯代乙酸更高的反应性,这归因于较低的CX键解离能。另一方面,尽管氯乙酸反应性随分子的氯化度而增加,特别是从一个到两个Cl取代基,但随着Br取代基的增加,溴乙酸没有显示出更高的反应速率。基于获得的结果,提出了通过逐步和/或协同反应的不同反应途径用于HAA的HDH。与这些途径相一致,还开发了动力学模型,可以成功地描述实验数据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号