Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dual Regioselective Targeting the Same Receptor in Nanoparticle-Mediated Combination Immuno/Chemotherapy for Enhanced Image-Guided Cancer Treatment.
ACS Nano ( IF 15.8 ) Pub Date : 2020-09-10 , DOI: 10.1021/acsnano.0c03421 Victoria O Shipunova 1, 2 , Elena N Komedchikova 1 , Polina A Kotelnikova 1 , Ivan V Zelepukin 1, 2 , Alexey A Schulga 1 , Galina M Proshkina 1 , Elena I Shramova 1 , Hilliard L Kutscher 3, 4, 5 , Georgij B Telegin 1 , Andrei V Kabashin 2, 6 , Paras N Prasad 2, 3 , Sergey M Deyev 1, 2
ACS Nano ( IF 15.8 ) Pub Date : 2020-09-10 , DOI: 10.1021/acsnano.0c03421 Victoria O Shipunova 1, 2 , Elena N Komedchikova 1 , Polina A Kotelnikova 1 , Ivan V Zelepukin 1, 2 , Alexey A Schulga 1 , Galina M Proshkina 1 , Elena I Shramova 1 , Hilliard L Kutscher 3, 4, 5 , Georgij B Telegin 1 , Andrei V Kabashin 2, 6 , Paras N Prasad 2, 3 , Sergey M Deyev 1, 2
Affiliation
|
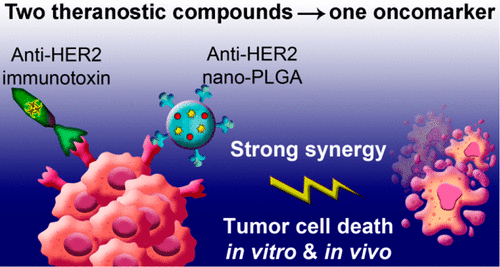
When combined with immunotherapy, image-guided targeted delivery of chemotherapeutic agents is a promising direction for combination cancer theranostics, but this approach has so far produced only limited success due to a lack of molecular targets on the cell surface and low therapeutic index of conventional chemotherapy drugs. Here, we demonstrate a synergistic strategy of combination immuno/chemotherapy in conditions of dual regioselective targeting, implying vectoring of two distinct binding sites of a single oncomarker (here, HER2) with theranostic compounds having a different mechanism of action. We use: (i) PLGA nanoformulation, loaded with an imaging diagnostic fluorescent dye (Nile Red) and a chemotherapeutic drug (doxorubicin), and functionalized with affibody ZHER2:342 (8 kDa); (ii) bifunctional genetically engineered DARP-LoPE (42 kDa) immunotoxin comprising of a low-immunogenic modification of therapeutic Pseudomonas exotoxin A (LoPE) and a scaffold targeting protein, DARPin9.29 (14 kDa). According to the proposed strategy, the first chemotherapeutic nanoagent is targeted by the affibody to subdomain III and IV of HER2 with 60-fold specificity compared with nontargeted particles, while the second immunotoxin is effectively targeted by DARPin molecule to subdomain I of HER2. We demonstrate that this dual targeting strategy can enhance anticancer therapy of HER2-positive cells with a very strong synergy, which made possible 1000-fold decrease of effective drug concentration in vitro and a significant enhancement of HER2 cancer therapy compared to monotherapy in vivo. Moreover, this therapeutic combination prevented the appearance of secondary tumor nodes. Thus, the suggested synergistic strategy utilizing dual targeting of the same oncomarker could give rise to efficient methods for aggressive tumors treatment.
中文翻译:

纳米区域介导的联合免疫/化学疗法中针对相同受体的双重区域选择性靶向,可增强图像指导的癌症治疗。
当与免疫疗法相结合时,以图像为指导的靶向化疗药物是组合癌症治疗学的有前途的方向,但是由于在细胞表面缺乏分子靶标并且常规化疗的治疗指数较低,因此该方法迄今为止仅取得了有限的成功毒品。在这里,我们展示了在双重区域选择性靶向的条件下联合免疫/化学疗法的协同策略,这意味着将单个oncomarker(此处为HER2)的两个不同结合位点与具有不同作用机理的治疗药物进行载体化。我们使用:(i)PLGA纳米制剂,装有成像诊断荧光染料(尼罗红)和化学治疗药物(阿霉素),并用亲和体Z HER2:342功能化(8 kDa);(ii)双功能基因工程DARP-LoPE(42 kDa)免疫毒素,包括治疗性假单胞菌外毒素A(LoPE)和支架靶向蛋白DARPin9.29(14 kDa)的低免疫原性修饰。根据提出的策略,与非靶向颗粒相比,第一种化学疗法纳米剂被亲和体靶向HER2的亚域III和IV,具有60倍的特异性,而第二种免疫毒素被DARPin分子有效靶向于HER2的亚域I。我们证明了这种双重靶向策略可以以非常强的协同作用增强HER2阳性细胞的抗癌治疗,从而使体外有效药物浓度降低1000倍成为可能与体内单一治疗相比,HER2癌症治疗显着增强。而且,这种治疗组合阻止了继发性肿瘤结节的出现。因此,建议的利用相同靶子的双重靶向的协同策略可以产生用于侵袭性肿瘤治疗的有效方法。
更新日期:2020-10-28
中文翻译:

纳米区域介导的联合免疫/化学疗法中针对相同受体的双重区域选择性靶向,可增强图像指导的癌症治疗。
当与免疫疗法相结合时,以图像为指导的靶向化疗药物是组合癌症治疗学的有前途的方向,但是由于在细胞表面缺乏分子靶标并且常规化疗的治疗指数较低,因此该方法迄今为止仅取得了有限的成功毒品。在这里,我们展示了在双重区域选择性靶向的条件下联合免疫/化学疗法的协同策略,这意味着将单个oncomarker(此处为HER2)的两个不同结合位点与具有不同作用机理的治疗药物进行载体化。我们使用:(i)PLGA纳米制剂,装有成像诊断荧光染料(尼罗红)和化学治疗药物(阿霉素),并用亲和体Z HER2:342功能化(8 kDa);(ii)双功能基因工程DARP-LoPE(42 kDa)免疫毒素,包括治疗性假单胞菌外毒素A(LoPE)和支架靶向蛋白DARPin9.29(14 kDa)的低免疫原性修饰。根据提出的策略,与非靶向颗粒相比,第一种化学疗法纳米剂被亲和体靶向HER2的亚域III和IV,具有60倍的特异性,而第二种免疫毒素被DARPin分子有效靶向于HER2的亚域I。我们证明了这种双重靶向策略可以以非常强的协同作用增强HER2阳性细胞的抗癌治疗,从而使体外有效药物浓度降低1000倍成为可能与体内单一治疗相比,HER2癌症治疗显着增强。而且,这种治疗组合阻止了继发性肿瘤结节的出现。因此,建议的利用相同靶子的双重靶向的协同策略可以产生用于侵袭性肿瘤治疗的有效方法。







































 京公网安备 11010802027423号
京公网安备 11010802027423号