当前位置:
X-MOL 学术
›
Aging Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Vascular amyloid accumulation alters the gabaergic synapse and induces hyperactivity in a model of cerebral amyloid angiopathy.
Aging Cell ( IF 8.0 ) Pub Date : 2020-09-10 , DOI: 10.1111/acel.13233 Pablo Cisternas 1, 2 , Xavier Taylor 1, 2 , Abigail Perkins 1, 2 , Orlando Maldonado 1, 2 , Elysabeth Allman 1, 2 , Ricardo Cordova 1, 2 , Yamil Marambio 1, 2 , Braulio Munoz 1, 3 , Taylor Pennington 1, 3 , Shunian Xiang 4 , Jie Zhang 4 , Ruben Vidal 1, 5 , Brady Atwood 1, 3 , Cristian A Lasagna-Reeves 1, 2
Aging Cell ( IF 8.0 ) Pub Date : 2020-09-10 , DOI: 10.1111/acel.13233 Pablo Cisternas 1, 2 , Xavier Taylor 1, 2 , Abigail Perkins 1, 2 , Orlando Maldonado 1, 2 , Elysabeth Allman 1, 2 , Ricardo Cordova 1, 2 , Yamil Marambio 1, 2 , Braulio Munoz 1, 3 , Taylor Pennington 1, 3 , Shunian Xiang 4 , Jie Zhang 4 , Ruben Vidal 1, 5 , Brady Atwood 1, 3 , Cristian A Lasagna-Reeves 1, 2
Affiliation
|
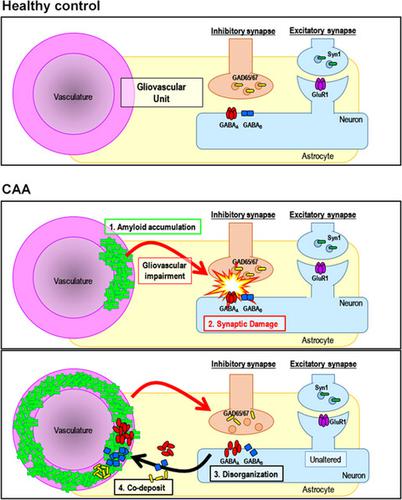
Cerebral amyloid angiopathy (CAA) is typified by the cerebrovascular deposition of amyloid. The mechanisms underlying the contribution of CAA to neurodegeneration are not currently understood. Although CAA is highly associated with the accumulation of β‐amyloid (Aβ), other amyloids are known to associate with the vasculature. Alzheimer's disease (AD) is characterized by parenchymal Aβ deposition and intracellular accumulation of tau as neurofibrillary tangles (NFTs), affecting synapses directly, leading to behavioral and physical impairment. CAA increases with age and is present in 70%–97% of individuals with AD. Studies have overwhelmingly focused on the connection between parenchymal amyloid accumulation and synaptotoxicity; thus, the contribution of vascular amyloid is mostly understudied. Here, synaptic alterations induced by vascular amyloid accumulation and their behavioral consequences were characterized using a mouse model of Familial Danish dementia (FDD), a neurodegenerative disease characterized by the accumulation of Danish amyloid (ADan) in the vasculature. The mouse model (Tg‐FDD) displays a hyperactive phenotype that potentially arises from impairment in the GABAergic synapses, as determined by electrophysiological analysis. We demonstrated that the disruption of GABAergic synapse organization causes this impairment and provided evidence that GABAergic synapses are impaired in patients with CAA pathology. Understanding the mechanism that CAA contributes to synaptic dysfunction in AD‐related dementias is of critical importance for developing future therapeutic interventions.
中文翻译:

在脑淀粉样血管病模型中,血管淀粉样蛋白的积累会改变伽巴能突触并诱导过度活跃。
脑淀粉样血管病 (CAA) 以淀粉样蛋白的脑血管沉积为代表。目前尚不清楚 CAA 对神经退行性变的贡献的机制。尽管 CAA 与 β-淀粉样蛋白 (Aβ) 的积累高度相关,但已知其他淀粉样蛋白与脉管系统相关。阿尔茨海默病 (AD) 的特征是实质 Aβ 沉积和 tau 细胞内积聚作为神经原纤维缠结 (NFT),直接影响突触,导致行为和身体损伤。CAA 随着年龄的增长而增加,并且存在于 70%–97% 的 AD 患者中。研究绝大多数集中在实质淀粉样蛋白积累和突触毒性之间的联系上。因此,血管淀粉样蛋白的贡献大多未被研究。这里,使用丹麦家族性痴呆 (FDD) 的小鼠模型表征由血管淀粉样蛋白积累引起的突触改变及其行为后果,FDD 是一种以丹麦淀粉样蛋白 (ADan) 在脉管系统中积累为特征的神经退行性疾病。通过电生理分析确定,小鼠模型 (Tg-FDD) 显示出可能由 GABA 能突触受损引起的过度活跃表型。我们证明 GABA 能突触组织的破坏会导致这种损伤,并提供证据表明 GABA 能突触在 CAA 病理患者中受损。了解 CAA 导致 AD 相关痴呆中突触功能障碍的机制对于开发未来的治疗干预至关重要。
更新日期:2020-10-23
中文翻译:

在脑淀粉样血管病模型中,血管淀粉样蛋白的积累会改变伽巴能突触并诱导过度活跃。
脑淀粉样血管病 (CAA) 以淀粉样蛋白的脑血管沉积为代表。目前尚不清楚 CAA 对神经退行性变的贡献的机制。尽管 CAA 与 β-淀粉样蛋白 (Aβ) 的积累高度相关,但已知其他淀粉样蛋白与脉管系统相关。阿尔茨海默病 (AD) 的特征是实质 Aβ 沉积和 tau 细胞内积聚作为神经原纤维缠结 (NFT),直接影响突触,导致行为和身体损伤。CAA 随着年龄的增长而增加,并且存在于 70%–97% 的 AD 患者中。研究绝大多数集中在实质淀粉样蛋白积累和突触毒性之间的联系上。因此,血管淀粉样蛋白的贡献大多未被研究。这里,使用丹麦家族性痴呆 (FDD) 的小鼠模型表征由血管淀粉样蛋白积累引起的突触改变及其行为后果,FDD 是一种以丹麦淀粉样蛋白 (ADan) 在脉管系统中积累为特征的神经退行性疾病。通过电生理分析确定,小鼠模型 (Tg-FDD) 显示出可能由 GABA 能突触受损引起的过度活跃表型。我们证明 GABA 能突触组织的破坏会导致这种损伤,并提供证据表明 GABA 能突触在 CAA 病理患者中受损。了解 CAA 导致 AD 相关痴呆中突触功能障碍的机制对于开发未来的治疗干预至关重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号