当前位置:
X-MOL 学术
›
Eur. J. Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Difluorochloronium(III) Fluoridometallates – from Molecular Building Blocks to (Helical) Chains
European Journal of Inorganic Chemistry ( IF 2.2 ) Pub Date : 2020-09-11 , DOI: 10.1002/ejic.202000845 Benjamin Scheibe 1 , Ralf Haiges 2 , Sergei I. Ivlev 1 , Antti J. Karttunen 3 , Ulrich Müller 1 , Karl O. Christe 2 , Florian Kraus 1
European Journal of Inorganic Chemistry ( IF 2.2 ) Pub Date : 2020-09-11 , DOI: 10.1002/ejic.202000845 Benjamin Scheibe 1 , Ralf Haiges 2 , Sergei I. Ivlev 1 , Antti J. Karttunen 3 , Ulrich Müller 1 , Karl O. Christe 2 , Florian Kraus 1
Affiliation
|
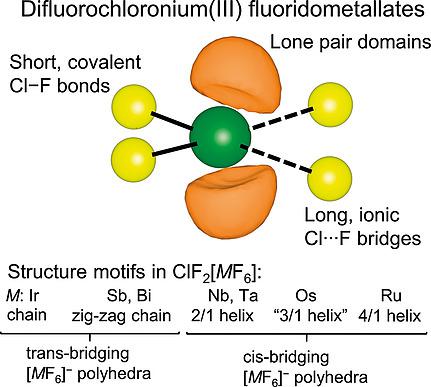
Difluorochloronium(III) compounds were synthesized from the reaction of metal powders (Ru, Os, Ir, Au), metal fluorides (NbF5, SbF3, BiF5) or a metal chloride (TaCl5) with excess liquid chlorine trifluoride. The compounds ClF2[AuF4], ClF2[MF6] (M = Nb, Ta, Ru, Os, Ir, Sb, Bi) and ClF2[Ta2F11] were obtained in crystalline form and their crystal structures were determined by single‐crystal X‐ray diffraction. The ClF2+ cations in the investigated compounds are bent, containing two strong, short, mainly covalent Cl–F bonds and two sterically active, free valence electron pairs in a pseudo‐tetrahedral arrangement. The coordination around the Cl atom is extended by two highly ionic, long fluorine bridges to neighboring fluoridometallate anions, resulting in a total coordination number of six. The crystal structures vary among the ClF2+ compounds and range from molecular building blocks, such as dimeric (ClF2[AuF4])2 and (ClF2[Ta2F11])2, to chains, some of which being helical, as in ClF2[MF6], (M = Nb, Ta, Ru, Os, Ir, Sb, Bi). Quantum‐chemical solid‐state and gas‐phase calculations were carried out to elucidate the bonding within the ClF2+ cations and their interactions with the bridging F atoms.
中文翻译:

二氟氯鎓(III)氟代金属盐-从分子构造单元到(螺旋)链
由金属粉末(Ru,Os,Ir,Au),金属氟化物(NbF 5,SbF 3,BiF 5)或金属氯化物(TaCl 5)与过量的液态三氟化氯反应合成二氟氯鎓(III )化合物。获得了结晶形式的化合物ClF 2 [AuF 4 ],ClF 2 [ M F 6 ](M = Nb,Ta,Ru,Os,Ir,Sb,Bi)和ClF 2 [Ta 2 F 11 ]。结构是通过单晶X射线衍射确定的。ClF 2 +被研究化合物中的阳离子是弯曲的,以假四面体排列包含两个强的,短的,主要是共价的Cl-F键和两个空间活性的自由价电子对。在Cl原子周围的配位被两个高度离子化的长氟桥延伸至相邻的氟代金属盐阴离子,因此配位总数为六个。晶体结构在ClF 2 +化合物之间有所不同,范围从分子构造单元,例如二聚(ClF 2 [AuF 4 ])2和(ClF 2 [Ta 2 F 11 ])2到链,其中一些是螺旋的,如ClF 2 [ MF 6 ],(M= Nb,Ta,Ru,Os,Ir,Sb,Bi)。进行了量子化学固态和气相计算,以阐明ClF 2 +阳离子内的键及其与桥连的F原子的相互作用。
更新日期:2020-09-11
中文翻译:

二氟氯鎓(III)氟代金属盐-从分子构造单元到(螺旋)链
由金属粉末(Ru,Os,Ir,Au),金属氟化物(NbF 5,SbF 3,BiF 5)或金属氯化物(TaCl 5)与过量的液态三氟化氯反应合成二氟氯鎓(III )化合物。获得了结晶形式的化合物ClF 2 [AuF 4 ],ClF 2 [ M F 6 ](M = Nb,Ta,Ru,Os,Ir,Sb,Bi)和ClF 2 [Ta 2 F 11 ]。结构是通过单晶X射线衍射确定的。ClF 2 +被研究化合物中的阳离子是弯曲的,以假四面体排列包含两个强的,短的,主要是共价的Cl-F键和两个空间活性的自由价电子对。在Cl原子周围的配位被两个高度离子化的长氟桥延伸至相邻的氟代金属盐阴离子,因此配位总数为六个。晶体结构在ClF 2 +化合物之间有所不同,范围从分子构造单元,例如二聚(ClF 2 [AuF 4 ])2和(ClF 2 [Ta 2 F 11 ])2到链,其中一些是螺旋的,如ClF 2 [ MF 6 ],(M= Nb,Ta,Ru,Os,Ir,Sb,Bi)。进行了量子化学固态和气相计算,以阐明ClF 2 +阳离子内的键及其与桥连的F原子的相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号