当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
4‐Sulfonyloxy/alkoxy benzoxazolone derivatives with high anti‐inflammatory activities: Synthesis, biological evaluation, and mechanims of action via p38/ERK‐NF‐κB/iNOS pathway
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2020-09-11 , DOI: 10.1111/cbdd.13784 Li Tang 1, 2 , Jie-Ran Luo 1 , Xiao-Yan Wang 1 , Bei Zhao 1 , Rui Ge 1 , Tai-Gang Liang 1 , Shu-Rong Ban 1 , Qing-Shan Li 1, 2
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2020-09-11 , DOI: 10.1111/cbdd.13784 Li Tang 1, 2 , Jie-Ran Luo 1 , Xiao-Yan Wang 1 , Bei Zhao 1 , Rui Ge 1 , Tai-Gang Liang 1 , Shu-Rong Ban 1 , Qing-Shan Li 1, 2
Affiliation
|
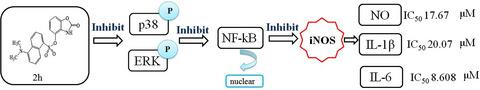
In an effort to discover new agents with high anti‐inflammatory activity, 22 new 4‐sulfonyloxy/alkoxy benzoxazolone derivatives were synthesized, characterized, and evaluated for their anti‐inflammatory activities against lipopolysaccharide (LPS)‐induced nitric oxide (NO) production and TNF‐α expression in RAW 264.7 cells in vitro. Most of these compounds displayed greater inhibitory ability against NO production than the lead compound 4‐o‐methyl‐benzenesulfonyl benzoxazolone, and the most active compound 2h exhibited the strongest inhibitory activity against NO, IL‐1β, and IL‐6 production with IC50 values 17.67, 20.07, and 8.61 μΜ, respectively. The effects of 2h were comparable or stronger than those of the positive control celecoxib. Compound 2h also displayed higher activity in vivo than celecoxib in a mouse model of xylene‐induced ear edema, based on their inhibitory rates of 42.69% and 30.87%, respectively. Further molecular analysis revealed that compound 2h significantly reduced the iNOS levels in cell supernatant and suppressed the protein expression of iNOS, p‐p38, p‐ERK, and nuclear NF‐κB. The results indicated that the anti‐inflammatory effect of 2h might be realized through the regulation of ERK‐ and p38‐mediated mitogen‐activated protein kinase (MAPK)‐NF‐κB/iNOS signaling, thereby reducing the excessive release of NO, IL‐1β, and IL‐6. Our findings demonstrated that compound 2h, a new benzoxazolone derivative, could inhibit activation of the MAPK‐NF‐κB/iNOS pathway, supporting its potential as a novel anti‐inflammatory agent.
中文翻译:

具有高抗炎活性的 4-磺酰氧基/烷氧基苯并恶唑酮衍生物:通过 p38/ERK-NF-κB/iNOS 途径的合成、生物学评价和作用机制
为了发现具有高抗炎活性的新药剂,合成、表征和评估了 22 种新的 4-磺酰氧基/烷氧基苯并恶唑酮衍生物对脂多糖 (LPS) 诱导的一氧化氮 (NO) 产生的抗炎活性和体外 RAW 264.7 细胞中的 TNF-α 表达。这些化合物中的大多数显示出比先导化合物 4 - o-甲基-苯磺酰基苯并恶唑酮更强的抑制 NO 产生的能力,活性最强的化合物2h对 NO、IL-1β 和 IL-6 产生的抑制活性最强,IC 50值分别为 17.67、20.07 和 8.61 μM。2h的效果与阳性对照塞来昔布相当或更强。在二甲苯诱导的耳水肿小鼠模型中,化合物2h 的体内活性也高于塞来昔布,抑制率分别为 42.69% 和 30.87%。进一步的分子分析表明,化合物2h显着降低了细胞上清液中的 iNOS 水平,并抑制了 iNOS、p-p38、p-ERK 和核 NF-κB 的蛋白质表达。结果表明2h的抗炎作用可能是通过调节ERK-和p38介导的丝裂原活化蛋白激酶(MAPK)-NF-κB/iNOS信号通路来实现的,从而减少NO、IL- 1β 和 IL-6。我们的研究结果表明,化合物2h一种新的苯并恶唑酮衍生物,可以抑制 MAPK-NF-κB/iNOS 通路的激活,支持其作为新型抗炎剂的潜力。
更新日期:2020-09-11
中文翻译:

具有高抗炎活性的 4-磺酰氧基/烷氧基苯并恶唑酮衍生物:通过 p38/ERK-NF-κB/iNOS 途径的合成、生物学评价和作用机制
为了发现具有高抗炎活性的新药剂,合成、表征和评估了 22 种新的 4-磺酰氧基/烷氧基苯并恶唑酮衍生物对脂多糖 (LPS) 诱导的一氧化氮 (NO) 产生的抗炎活性和体外 RAW 264.7 细胞中的 TNF-α 表达。这些化合物中的大多数显示出比先导化合物 4 - o-甲基-苯磺酰基苯并恶唑酮更强的抑制 NO 产生的能力,活性最强的化合物2h对 NO、IL-1β 和 IL-6 产生的抑制活性最强,IC 50值分别为 17.67、20.07 和 8.61 μM。2h的效果与阳性对照塞来昔布相当或更强。在二甲苯诱导的耳水肿小鼠模型中,化合物2h 的体内活性也高于塞来昔布,抑制率分别为 42.69% 和 30.87%。进一步的分子分析表明,化合物2h显着降低了细胞上清液中的 iNOS 水平,并抑制了 iNOS、p-p38、p-ERK 和核 NF-κB 的蛋白质表达。结果表明2h的抗炎作用可能是通过调节ERK-和p38介导的丝裂原活化蛋白激酶(MAPK)-NF-κB/iNOS信号通路来实现的,从而减少NO、IL- 1β 和 IL-6。我们的研究结果表明,化合物2h一种新的苯并恶唑酮衍生物,可以抑制 MAPK-NF-κB/iNOS 通路的激活,支持其作为新型抗炎剂的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号