当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of i-Corona[6]arenes for Selective Anion Binding: Interdependent and Synergistic Anion-π and Hydrogen Bond Interactions.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-09-10 , DOI: 10.1002/anie.202008997 Sheng-Yi Zhuang 1 , Ying Cheng 1 , Qian Zhang 2 , Shuo Tong 2 , Mei-Xiang Wang 2
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-09-10 , DOI: 10.1002/anie.202008997 Sheng-Yi Zhuang 1 , Ying Cheng 1 , Qian Zhang 2 , Shuo Tong 2 , Mei-Xiang Wang 2
Affiliation
|
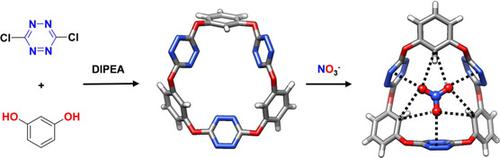
i‐Corona[3]arene[3]tetrazines were synthesized from the nucleophilic aromatic substitution reaction of resorcinol and its derivatives with 3,6‐dichlorotetrazine in a one‐pot fashion under mild conditions. All of the resulting macrocycles adopted 1,3,5‐alternate conformation irrespective of the nature of the substituents on both upper‐ and lower‐rims. i‐Corona[3]arene[3]tetrazine was found to self‐regulate its macrocyclic conformation and cavity to recognize anions with binding constants spanning from 26 M−1 to 2.2×103 M−1 depending on the structure of the anions. The selective binding resulted from a significant interdependent and synergistic effect between multiple tetrazine π/anion and Caryl–H/anion hydrogen bond interactions. Taking advantage of synergistic effect revealed, a cyanobenzene‐embedded i‐corona[3]arene[3]tetrazine was designedly synthesized and highly selective and very strong affinity toward nitrate with a binding constant of 2.2×105 M−1 was achieved.
中文翻译:

选择性阴离子结合的i-Corona [6]芳烃的合成:相互依赖和协同的阴离子π和氢键相互作用。
间苯二酚及其衍生物与3,6-二氯四嗪在温和条件下的亲核芳香取代反应合成了i-Corona [3] arene [3]四嗪。不论上缘和下缘的取代基性质如何,所有产生的大环均采用1,3,5-交替构象。发现i-Corona [3] arene [3]四嗪能够自我调节其大环构象和空腔,以识别结合常数介于26 M -1至2.2×10 3 M -1的阴离子,具体取决于阴离子的结构。选择性结合是由于多个四嗪π/阴离子与C芳基之间的显着相互依赖性和协同作用所致-H /阴离子氢键相互作用。利用所揭示的协同作用,设计合成了氰基苯嵌入的异氰酸酯[3]芳烃[3]四嗪,并获得了高选择性和对硝酸盐的非常强的亲和力,结合常数为2.2×10 5 M -1。
更新日期:2020-09-10
中文翻译:

选择性阴离子结合的i-Corona [6]芳烃的合成:相互依赖和协同的阴离子π和氢键相互作用。
间苯二酚及其衍生物与3,6-二氯四嗪在温和条件下的亲核芳香取代反应合成了i-Corona [3] arene [3]四嗪。不论上缘和下缘的取代基性质如何,所有产生的大环均采用1,3,5-交替构象。发现i-Corona [3] arene [3]四嗪能够自我调节其大环构象和空腔,以识别结合常数介于26 M -1至2.2×10 3 M -1的阴离子,具体取决于阴离子的结构。选择性结合是由于多个四嗪π/阴离子与C芳基之间的显着相互依赖性和协同作用所致-H /阴离子氢键相互作用。利用所揭示的协同作用,设计合成了氰基苯嵌入的异氰酸酯[3]芳烃[3]四嗪,并获得了高选择性和对硝酸盐的非常强的亲和力,结合常数为2.2×10 5 M -1。










































 京公网安备 11010802027423号
京公网安备 11010802027423号