Metabolic Engineering ( IF 6.8 ) Pub Date : 2020-09-11 , DOI: 10.1016/j.ymben.2020.09.006 Jiawei Ge 1 , Xiaohong Yang 2 , Hongwei Yu 1 , Lidan Ye 1
|
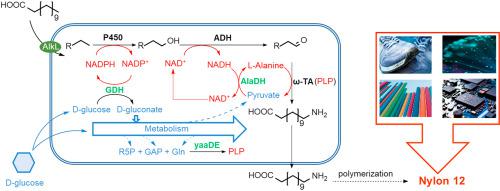
Biosynthesis of Nylon 12 monomer using dodecanoic acid (DDA) or its esters as the renewable feedstock typically involves ω-hydroxylation, oxidation and ω-amination. The dependence of hydroxylation and oxidation-catalyzing enzymes on redox cofactors, and the requirement of L-alanine as the co-substrate and pyridoxal 5′-phosphate (PLP) as the coenzyme for transamination, raise the issue of redox imbalance and cofactor shortage, challenging the development of efficient biocatalysts. Simultaneous regeneration of the redox equivalents, PLP and L-alanine required in the artificial pathway was enabled by its interfacing with the native metabolism of the host using glucose dehydrogenase (GDH), L-alanine dehydrogenase (AlaDH) and an exogenous ribose 5-phosphate (R5P)-dependent PLP synthesis pathway as bridges. Further engineering of the host by blocking β-oxidation and enhancing substrate uptake improved the ω-aminododecanoic acid (ω-AmDDA) yield to 96.5%. This study offers a strategy to resolve the cofactor imbalance issue commonly encountered in whole-cell biocatalysis and meanwhile lays a solid foundation for Nylon 12 bioproduction.
中文翻译:

尼龙 12 单体的高产全细胞生物合成,多种辅助因子的自给自足供应。
使用十二烷酸 (DDA) 或其酯作为可再生原料的尼龙 12 单体的生物合成通常涉及 ω-羟基化、氧化和 ω-胺化。羟基化和氧化催化酶对氧化还原辅因子的依赖性,以及需要 L-丙氨酸作为共底物和 5'-磷酸吡哆醛 (PLP) 作为转氨酶的辅酶,提出了氧化还原失衡和辅因子短缺的问题,挑战高效生物催化剂的开发。通过使用葡萄糖脱氢酶 (GDH)、L-丙氨酸脱氢酶 (AlaDH) 和外源性 5-磷酸核糖与宿主的天然代谢相结合,可以同时再生人工途径中所需的氧化还原当量、PLP 和 L-丙氨酸(R5P) 依赖性 PLP 合成途径作为桥梁。通过阻断 β-氧化和增强底物吸收来进一步改造宿主,将 ω-氨基十二烷酸 (ω-AmDDA) 的产率提高到 96.5%。该研究为解决全细胞生物催化中常见的辅因子失衡问题提供了一种策略,同时为尼龙12的生物生产奠定了坚实的基础。









































 京公网安备 11010802027423号
京公网安备 11010802027423号