Journal of Energy Chemistry ( IF 14.0 ) Pub Date : 2020-09-11 , DOI: 10.1016/j.jechem.2020.09.005 Di Wang , Shanshan Qiao , Jia Guo , Yuan Guo , Qian Xu , Naeem Akram , Jide Wang
|
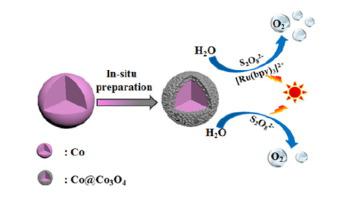
The photocatalytic performances of water oxidation were usually carried out in two different systems, photosensitizer and non-photosensitizer systems. There is few report about the same catalyst used in two systems and therefore it is of great significant to compare its role of the same catalyst in two systems and explore its different reaction mechanisms. In this work, first 4 kinds of metallic Co microparticles were obtained by different reduction methods through hydrothermal processes, and Co@Co3O4 core-shell microparticles (1–4) were obtained from these metallic Co microparticles oxidized in air or in the reacting solution in situ. The core-shell structure of 1 was characterized by a series of analytical techniques. 1–4 exhibited excellent activities and stabilities in the [Ru(bpy)3]2+/S2O82−/light system when they were used as catalysts for the photocatalytic water oxidation. The maximum O2 evolution of 1 after 20 min’s illumination was 98.2 μmol, the O2 yield was 65.5%, the initial turnover frequency was 6.6 × 10−3, the initial quantum efficiency (ΦQY initial) was 15.0% higher than Co (8.3%), CoO (11.7%), Co2O3 (1.2%), Co3O4 (2.8%) and 5 (2.2%). Even after the sixth run, the catalytic activity of recovered 1 still remained 85.1% of initial activity. In addition, the photocatalytic performances of 1 in the [Ru(bpy)3]2+/S2O82−/light system and S2O82−/light system were compared for the first time. In the non-photosensitizer system, 1 shows bifunctional roles and acts as optical absorption center and active catalytic site, and oxygen evolution rate is lower and it takes longer time. In the photosensitizer system, 1 only acts as a catalyst, the photosensitizer enhances the light absorption and promotes water oxidation reaction with higher O2 yield and QE, meanwhile the photosensitizer brings the defect of high cost and instability into the system. Based on the results the two different reaction mechanisms were deeply discussed.
中文翻译:

用于光催化水氧化的高效Co @ Co3O4核壳催化剂及其在两种不同光催化体系中的行为
水氧化的光催化性能通常在两种不同的系统中进行,即光敏剂和非光敏剂系统。关于在两个系统中使用相同催化剂的报道很少,因此,比较相同催化剂在两个系统中的作用并探索其不同的反应机理具有重大意义。在这项工作中,通过水热法通过不同的还原方法获得了前4种金属Co微粒,以及Co @ Co 3 O 4。从这些金属Co微粒在空气中或在原位反应溶液中被氧化后得到核-壳微粒(1-4)。1的核-壳结构通过一系列分析技术来表征。当它们用作光催化水氧化的催化剂时,[1-4 ]在[Ru(bpy)3 ] 2+ / S 2 O 8 2- /光体系中表现出出色的活性和稳定性。的最大直径:2 1后20分钟的照明的进化为98.2微摩尔时,O 2产率是65.5%,初始转换频率为6.6×10 -3,初始量子效率(Φ QY初始)比Co(8.3%),CoO(11.7%),Co 2 O 3(1.2%),Co 3 O 4(2.8%)和5(2.2%)高15.0%。即使在第六次运行后,回收的1的催化活性仍保持初始活性的85.1%。另外,[Ru(bpy)3 ] 2+ / S 2 O 8 2- /光体系和S 2 O 8 2-的光催化性能为1。/ light系统进行了第一次比较。在非光敏剂体系中,1表现出双功能作用,并充当光吸收中心和活性催化部位,并且氧的释放速率较低,并且花费的时间更长。在光敏剂体系中,1仅用作催化剂,光敏剂以较高的O 2产率和QE增强光吸收并促进水氧化反应,同时光敏剂给系统带来了成本高和不稳定的缺点。基于结果,深入讨论了两种不同的反应机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号