Journal of Allergy and Clinical Immunology ( IF 14.2 ) Pub Date : 2020-09-10 , DOI: 10.1016/j.jaci.2020.08.033 Yan Zhang 1 , Danh C Do 2 , Xinyue Hu 1 , Ji Wang 3 , Yilin Zhao 4 , Sumita Mishra 5 , Xin Zhang 3 , Mei Wan 6 , Peisong Gao 2
|
Background
Autophagy plays an important role in causing inflammatory responses initiated by environmental pollutants and respiratory tract infection.
Objective
We sought to investigate the role of cockroach allergen–induced excessive activation of autophagy in allergic airway inflammation and its underlying molecular mechanisms.
Methods
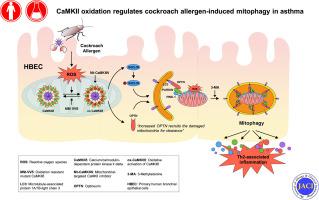
Environmental allergen–induced autophagy was investigated in the primary human bronchial epithelial cells (HBECs) and lung tissues of asthmatic mouse model and patients. The role of autophagy in asthma development was examined by using autophagy inhibitor 3-methyladenine in an asthma mouse model. Furthermore, the involvements of reactive oxygen species (ROS) and oxidized Ca2+/calmodulin-dependent protein kinase II (ox-CaMKII) signaling in regulating autophagy during asthma were examined in allergen-treated HBECs and mouse model.
Results
Cockroach allergen activated autophagy in HBECs and in the lung tissues from asthmatic patients and mice. Autophagy inhibitor 3-methyladenine significantly attenuated airway hyperresponsiveness, TH2-associated lung inflammation, and ROS generation. Mechanistically, we demonstrated a pathological feedforward circuit between cockroach allergen–induced ROS and autophagy that is mediated through CaMKII oxidation. Furthermore, transgenic mice with ROS-resistant CaMKII MM-VVδ showed attenuation of TH2-associated lung inflammation and autophagy. Mitochondrial ox-CaMKII inhibition induced by adenovirus carrying mitochondrial-targeted inhibitor peptide CaMKIIN suppresses cockroach allergen–induced autophagy, mitochondrial dysfunction, mitophagy, and cytokine production in HBECs. Finally, mitochondrial CaMKII inhibition suppressed the expression of one of the key ubiquitin-binding autophagy receptors, optineurin, and its recruitment to fragmented mitochondria. Optineurin knockdown inhibited cockroach allergy–induced mitophagy.
Conclusions
Our data suggest a previously uncovered axis of allergen-ROS-ox-CaMKII-mitophagy in the development of allergic airway inflammation and asthma.
中文翻译:

CaMKII氧化调节蟑螂过敏原诱导的哮喘线粒体自噬
背景
自噬在引起由环境污染物和呼吸道感染引发的炎症反应中起重要作用。
客观的
我们试图研究蟑螂过敏原诱导的自噬过度激活在过敏性气道炎症中的作用及其潜在的分子机制。
方法
在哮喘小鼠模型和患者的原代人支气管上皮细胞 (HBEC) 和肺组织中研究了环境过敏原诱导的自噬。通过在哮喘小鼠模型中使用自噬抑制剂 3-甲基腺嘌呤来检查自噬在哮喘发展中的作用。此外,在过敏原处理的 HBEC 和小鼠模型中检测了活性氧 (ROS) 和氧化的 Ca 2+ /钙调蛋白依赖性蛋白激酶 II (ox-CaMKII) 信号传导在调节哮喘期间自噬中的作用。
结果
蟑螂过敏原激活 HBEC 和哮喘患者和小鼠肺组织中的自噬。自噬抑制剂 3-甲基腺嘌呤显着减弱气道高反应性、TH 2相关的肺部炎症和 ROS 的产生。从机制上讲,我们证明了蟑螂过敏原诱导的 ROS 和通过 CaMKII 氧化介导的自噬之间的病理前馈回路。此外,具有 ROS 抗性 CaMKII MM-VVδ 的转基因小鼠显示 T H减弱2 相关的肺部炎症和自噬。携带线粒体靶向抑制肽 CaMKIIN 的腺病毒诱导的线粒体 ox-CaMKII 抑制可抑制 HBECs 中蟑螂过敏原诱导的自噬、线粒体功能障碍、线粒体自噬和细胞因子的产生。最后,线粒体 CaMKII 抑制抑制了一种关键的泛素结合自噬受体 optineurin 的表达,并抑制了它向碎片化线粒体的募集。Optineurin 敲低抑制了蟑螂过敏诱导的线粒体自噬。
结论
我们的数据表明以前发现的过敏原-ROS-ox-CaMKII-线粒体自噬在过敏性气道炎症和哮喘的发展中的作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号