Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-09-11 , DOI: 10.1016/j.bioorg.2020.104273 Jie Yang 1 , Wen-Wen Mu 1 , Yu-Xin Cao 1 , Guo-Yun Liu 1
|
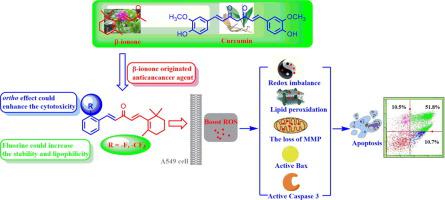
β-ionone, a cyclic terpenoid compound present in many fruits, has been showed a broad spectrum of biological activities. In this paper, we synthesized a panel of β-ionone derivatives and tested their anti-proliferation activity on cancer cell by the MTT assay. The results showed that most of the β-ionone derivatives were more active than β-ionone and curcumin. Particularly, the β-ionone derivatives (1a, 1d and 1g) with ortho-substituents on the aromatic ring exhibited much stronger cytotoxicity than their corresponding meta- and para-substituted compounds. Importantly, the cytotoxicity of the β-ionone derivatives (1a, 1d and 1g) were relationship with their reactive oxygen species (ROS)-generation abilities, which could lead to the redox imbalance, lipid peroxidation, the loss of mitochondrial membrane potential (MMP), the activation of Bax and Caspase 3, followed by cell apoptosis. This work suggest that the “ortho effect”, the ROS-generation ability and drawing fluorine atom into drugs may play a potent role in enhancing the anticancer activity of β-ionone derivatives.
中文翻译:

通过增加ROS的生成和β-紫罗兰酮定向促凋亡剂的生物学评估。
β-紫罗兰酮是许多水果中存在的环状萜类化合物,已显示出广泛的生物活性。在本文中,我们合成了一组β-紫罗兰酮衍生物,并通过MTT法测试了它们对癌细胞的抗增殖活性。结果表明,大多数β-紫罗兰酮衍生物比β-紫罗兰酮和姜黄素具有更高的活性。特别地,在芳环上具有邻取代基的β-紫罗兰酮衍生物(1a,1d和1g)比其相应的间位和对位取代的化合物表现出更强的细胞毒性。重要的是,β-紫罗兰酮衍生物(1a,1d和1g)与其活性氧(ROS)生成能力有关,可能导致氧化还原失衡,脂质过氧化,线粒体膜电位(MMP)丧失,Bax和Caspase 3活化以及随后的细胞凋亡。这项工作表明,“邻位效应”,ROS生成能力以及将氟原子吸纳到药物中可能在增强β-紫罗兰酮衍生物的抗癌活性方面发挥了重要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号