Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2020-09-11 , DOI: 10.1016/j.bmcl.2020.127551 Ulrich Grädler 1 , Michael Busch 1 , Birgitta Leuthner 1 , Michael Raba 2 , Lars Burgdorf 1 , Martin Lehmann 1 , Nina Linde 1 , Christina Esdar 1
|
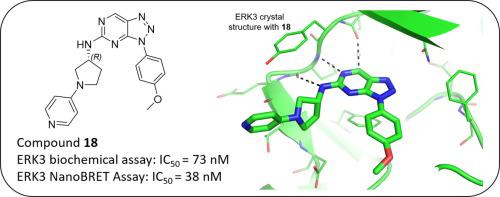
Triazolo[4,5-d]pyrimidin-5-amines were identified from kinase selectivity screening as novel ERK3 inhibitors with sub-100 nanomolar potencies in a biochemical assay using MK5 as substrate and with an attractive kinase selectivity profile. ERK3 crystal structures clarified the inhibitor binding mode in the ATP pocket with impact on A-loop, GC-loop and αC-helix conformations suggesting a potential structural link towards MK5 interaction via the FHIEDE motif. The inhibitors also showed sub-100 nM potencies in a cellular ERK3 NanoBRET assay and with excellent correlation to the biochemical IC50s. This novel series provides valuable tool compounds to further investigate the biological function and activation mechanism of ERK3.
中文翻译:

新型选择性 ERK3 抑制剂的生化、细胞和结构表征。
Triazolo[4,5 - d ]pyrimidin-5-amines 从激酶选择性筛选中被鉴定为新型 ERK3 抑制剂,在使用 MK5 作为底物的生化测定中具有低于 100 纳摩尔的效力,并具有有吸引力的激酶选择性特征。ERK3 晶体结构阐明了 ATP 袋中的抑制剂结合模式,对 A 环、GC 环和 αC 螺旋构象的影响表明通过 FHIEDE 基序与 MK5 相互作用存在潜在的结构联系。这些抑制剂还在细胞 ERK3 NanoBRET 测定中显示出低于 100 nM 的效力,并且与生化 IC 50 s具有极好的相关性。这个新系列为进一步研究 ERK3 的生物学功能和激活机制提供了有价值的工具化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号