Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-09-11 , DOI: 10.1016/j.bmc.2020.115758 Robert D Barrows 1 , Jared T Hammill 2 , Michael C Tran 1 , Mofolusho O Falade 2 , Amy L Rice 2 , Christopher W Davis 1 , Thomas J Emge 1 , Paul R Rablen 3 , R Kiplin Guy 2 , Spencer Knapp 1
|
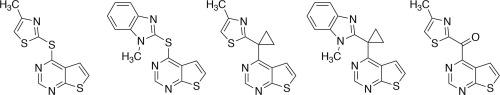
The 4-(heteroarylthio)thieno[2,3-d]pyrimidine (TTP) series of antimalarials, represented by 1 and 17, potently inhibit proliferation of the 3D7 strain of P. falciparum (EC50 70–100 nM), but suffer from oxidative metabolism. The 1,1-cyclopropylidene isosteres 6 and 16 were designed to obviate this drawback. They were prepared by a short route that features a combined Peterson methylenation / cyclopropanation transformation of, e. g., ketone 7. Isosteres 6 and 16 possess significantly attenuated antimalarial potency relative to parents 1 and 17. This outcome can be rationalized based on the increased out-of-plane steric demands of the latter two. In support of this hypothesis, the relatively flat ketone 7 retains some of the potency of 1, even though it appears to be a comparatively inferior mimic with respect to electronics and bond lengths and angles. We also demonstrate crystallographically and computationally an apparent increase in the strength of the intramolecular sulfur hole interaction of 1 upon protonation.
中文翻译:

在4-硫代噻吩并嘧啶(TTP)系列抗疟药中评估1,1-环亚丙基作为硫醚等排物
以1和17表示的4-(杂芳硫基)噻吩并[2,3- d ]嘧啶(TTP)系列抗疟药有效抑制恶性疟原虫3D7菌株的增殖(EC 50 70–100 nM),但受到影响来自氧化代谢。设计1,1-环亚丙基等排物6和16以消除该缺点。它们是通过短途径制备的,所述短途径具有例如酮7的结合的彼得森甲基化/环丙烷化转化的特征。相对于父母1和17,等位基因6和16具有显着减弱的抗疟疾效力。可以根据后两个方面增加的平面外空间需求来合理化此结果。为了支持该假设,相对平坦的酮7保留了1的某些效力,即使就电子学,键合长度和角度而言,酮7的模拟性相对较差。我们还证明了晶体学和计算上质子化时分子内硫空穴相互作用1的强度明显增加。











































 京公网安备 11010802027423号
京公网安备 11010802027423号