Frontiers of Chemical Science and Engineering ( IF 4.3 ) Pub Date : 2020-09-11 , DOI: 10.1007/s11705-020-1929-6 Vincent Froidevaux , Mélanie Decostanzi , Abdelatif Manseri , Sylvain Caillol , Bernard Boutevin , Rémi Auvergne
|
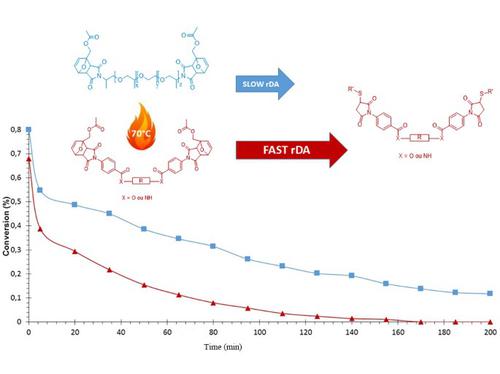
This study focuses on the synthesis of new liquid aromatic bismaleimide monomers in order to improve self-curing on demand (SCOD) systems previously based on aliphatic bismaleimides. These SCOD systems are based on Diels-Alder (DA)/retro-DA reactions. The syntheses of new different aromatic bismaleimides with ester and amide bonds are presented. These maleimides have been protected using DA reaction and characterized by 1H NMR analysis to determine protection rate and diastereomer ratios. The retro-DA reactions of both aromatic and aliphatic DA adducts in presence of thiol molecules were studied. Kinetic analysis was monitored by 1H NMR and compared to model study. Finally, both aromatic and aliphatic bismaleimides-based polymers were synthesized with 2-mercaptoethyl ether and thermal properties of polymers were compared. The glass transition temperature values ranged from −20 °C to 14 °C and very good thermal stabilities were observed (up to 300 °C).
中文翻译:

逆Diels-Alder反应引发的芳香族双马来酰亚胺与硫醇的“按需固化”改善
这项研究集中于合成新型液态芳族双马来酰亚胺单体,以改善以前基于脂族双马来酰亚胺的按需自固化(SCOD)系统。这些SCOD系统基于Diels-Alder(DA)/ retro-DA反应。介绍了具有酯键和酰胺键的新的不同芳族双马来酰亚胺的合成。这些马来酰亚胺已使用DA反应进行了保护,并通过1 H NMR分析确定了保护率和非对映异构体比率。研究了在硫醇分子存在下芳族和脂族DA加合物的逆DA反应。动力学分析由1监控1 H NMR和与模型研究比较。最后,用2-巯基乙基醚合成了芳香族和脂肪族双马来酰亚胺基聚合物,并比较了聚合物的热性能。玻璃化转变温度范围为-20°C至14°C,观察到非常好的热稳定性(最高300°C)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号