Current Topics in Medicinal Chemistry ( IF 2.9 ) Pub Date : 2020-08-31 , DOI: 10.2174/1568026620666200819155503 Martin Krátký 1 , Šárka Štěpánková 2 , Michaela Brablíková 3 , Katarína Svrčková 2 , Markéta Švarcová 1, 4 , Jarmila Vinšová 1
|
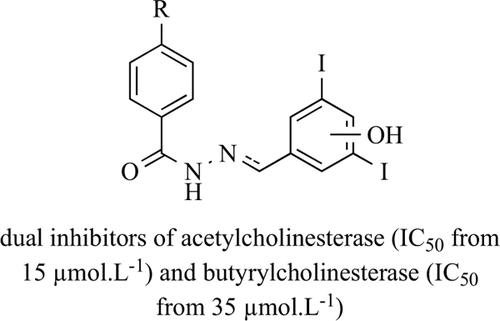
Background: Hydrazide-hydrazones have been known as scaffold with various biological activities including inhibition of acetyl- (AChE) and butyrylcholinesterase (BuChE). Cholinesterase inhibitors are mainstays of dementias’ treatment.
Objective: Twenty-five iodinated hydrazide-hydrazones and their analogues were designed as potential central AChE and BuChE inhibitors.
Methods: Hydrazide-hydrazones were synthesized from 4-substituted benzohydrazides and 2-/4- hydroxy-3,5-diiodobenzaldehydes. The compounds were investigated in vitro for their potency to inhibit AChE from electric eel and BuChE from equine serum using Ellman’s method. We calculated also physicochemical and structural parameters for CNS delivery.
Results: The derivatives exhibited a moderate dual inhibition with IC50 values ranging from 15.1-140.5 and 35.5 to 170.5 μmol.L-1 for AChE and BuChE, respectively. Generally, the compounds produced a balanced or more potent inhibition of AChE. N'-[(E)-(4-Hydroxy-3,5-diiodophenyl)methylidene]-4- nitrobenzohydrazide 2k and 4-fluoro-N'-(2-hydroxy-3,5-diiodobenzyl)benzohydrazide 3a were the most potent inhibitors of AChE and BuChE, respectively. Structure-activity relationships were established, and molecular docking studies confirmed interaction with enzymes.
Conclusion: Many novel hydrazide-hydrazones showed lower IC50 values than rivastigmine against AChE and some of them were comparable for BuChE to this drug used for the treatment of dementia. They interact with cholinesterases via non-covalent binding into the active site. Based on the BOILEDEgg approach, the majority of the derivatives met the criteria for blood-brain-barrier permeability.
中文翻译:

新型碘化肼-及其类似物,为乙酰基和丁酰胆碱酯酶抑制剂。
背景:肼-被称为具有多种生物活性的支架,包括抑制乙酰基(AChE)和丁酰胆碱酯酶(BuChE)。胆碱酯酶抑制剂是痴呆症治疗的支柱。
目的:设计二十五个碘化酰肼-及其类似物作为潜在的中枢AChE和BuChE抑制剂。
方法:由4-取代的苯甲酰肼和2- / 4-羟基-3,5-二碘联苯甲醛合成酰肼。使用Ellman方法在体外研究了这些化合物抑制电鳗中AChE和抑制马血清中BuChE的效力。我们还计算了中枢神经系统递送的理化和结构参数。
结果:衍生物对AChE和BuChE表现出中等双重抑制作用,IC50值分别为15.1-140.5和35.5至170.5μmol.L-1。通常,该化合物产生对AChE的平衡或更有效的抑制。N'-[(E)-(4-羟基-3,5-二碘苯基)亚甲基] -4-硝基苯并肼2k和4-氟-N'-(2-羟基-3,5-二碘苄基)苯并肼3a最AChE和BuChE的有效抑制剂。建立了构效关系,并且分子对接研究证实了与酶的相互作用。
结论:许多新颖的酰肼类药物对AChE的IC50值均低于rivastigmine,而且其中的BuChE与用于痴呆的药物相当。它们通过非共价结合进入活性位点与胆碱酯酶相互作用。基于BOILEDEgg方法,大多数衍生物符合血脑屏障通透性标准。











































 京公网安备 11010802027423号
京公网安备 11010802027423号