当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modeling Biologically Important NH···π Interactions Using peri-Disubstituted Naphthalenes.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-09-10 , DOI: 10.1021/acs.joc.0c01697 Alexander F Pozharskii 1 , Olga V Dyablo 1 , Olga G Pogosova 1 , Valery A Ozeryanskii 1 , Aleksander Filarowski 2 , Kseniya M Vasilikhina 1 , Narek A Dzhangiryan 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-09-10 , DOI: 10.1021/acs.joc.0c01697 Alexander F Pozharskii 1 , Olga V Dyablo 1 , Olga G Pogosova 1 , Valery A Ozeryanskii 1 , Aleksander Filarowski 2 , Kseniya M Vasilikhina 1 , Narek A Dzhangiryan 1
Affiliation
|
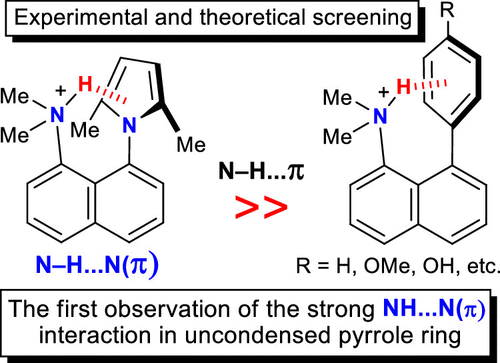
For the first time, systematic studies of 8-aryl and 8-pyrrolyl derivatives of 1-aminonaphthalene as simple, synthetically available, and nicely preorganized models were conducted for a better understanding the properties of NH···π interactions involved in the stabilization of the secondary and tertiary protein structures as well as the recognition of guest molecules by biological receptors. It was shown that the NH···π binding is especially effective when the NH-donor is a positively charged group, for example, Me2NH+, and the π-donor is an electron-rich aromatic substituent, in particular, the 1-pyrrolyl or the 4-hydroxyphenyl group. Using protonated tetrafluoroborate salts, a strong counterion effect was demonstrated by means of theoretical calculations. Through several mechanisms, including short CH···F contacts, bifurcation, and long-range dispersion, the counterion promotes considerable structural changes and weakens the NH···π interactions from 12–15 kcal mol–1 in “naked” cations to 5–9 kcal mol–1 in the salts. To this end, 8-(2,5-dimethylpyrrol-1-yl)-N,N-dimethylnaphthalene-1-ammonium tetrafluoroborate, with the record linearity and shortness (2.07 Å) of the NH···π-centroid bond, was recognized as the most appropriate model with the strongest NH···π interaction ever described.
中文翻译:

使用间二取代萘对生物学上重要的NH··π相互作用进行建模。
首次系统地研究了1-氨基萘的8-芳基和8-吡咯基衍生物作为简单,可合成并具有良好预组织的模型,以更好地了解参与稳定N的NH···π相互作用的性质。二级和三级蛋白质结构以及生物受体对客体分子的识别。结果表明,当NH-给体为带正电的基团,例如Me 2 NH +时,NH···π结合特别有效。,并且π-给体是富电子的芳族取代基,特别是1-吡咯基或4-羟苯基。使用质子化的四氟硼酸盐,通过理论计算证明了强大的抗衡离子作用。通过几种机制,包括短CH···F接触,分叉和远距离分散,抗衡离子促进了相当大的结构变化,并削弱了“裸”阳离子中12-15 kcal mol –1的NH···π相互作用。盐中5–9 kcal mol –1。为此,8-(2,5-二甲基吡咯-1-基)-N,N具有记录的NH··π质心键的线性和短度(2.07Å)的-二甲基萘-1-四氟硼酸铵被认为是有史以来最强的NH··π相互作用的最合适模型。
更新日期:2020-10-02
中文翻译:

使用间二取代萘对生物学上重要的NH··π相互作用进行建模。
首次系统地研究了1-氨基萘的8-芳基和8-吡咯基衍生物作为简单,可合成并具有良好预组织的模型,以更好地了解参与稳定N的NH···π相互作用的性质。二级和三级蛋白质结构以及生物受体对客体分子的识别。结果表明,当NH-给体为带正电的基团,例如Me 2 NH +时,NH···π结合特别有效。,并且π-给体是富电子的芳族取代基,特别是1-吡咯基或4-羟苯基。使用质子化的四氟硼酸盐,通过理论计算证明了强大的抗衡离子作用。通过几种机制,包括短CH···F接触,分叉和远距离分散,抗衡离子促进了相当大的结构变化,并削弱了“裸”阳离子中12-15 kcal mol –1的NH···π相互作用。盐中5–9 kcal mol –1。为此,8-(2,5-二甲基吡咯-1-基)-N,N具有记录的NH··π质心键的线性和短度(2.07Å)的-二甲基萘-1-四氟硼酸铵被认为是有史以来最强的NH··π相互作用的最合适模型。











































 京公网安备 11010802027423号
京公网安备 11010802027423号