当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Method for the Large-Scale Synthesis of Multifunctional 1,4-Dihydro-pyrrolo[3,2-b]pyrroles.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-09-09 , DOI: 10.1021/acs.joc.0c01665 Mariusz Tasior 1 , Olena Vakuliuk 1 , Daiki Koga 1 , Beata Koszarna 1 , Krzysztof Górski 1 , Marek Grzybowski 1 , Łukasz Kielesiński 1 , Maciej Krzeszewski 1 , Daniel T Gryko 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-09-09 , DOI: 10.1021/acs.joc.0c01665 Mariusz Tasior 1 , Olena Vakuliuk 1 , Daiki Koga 1 , Beata Koszarna 1 , Krzysztof Górski 1 , Marek Grzybowski 1 , Łukasz Kielesiński 1 , Maciej Krzeszewski 1 , Daniel T Gryko 1
Affiliation
|
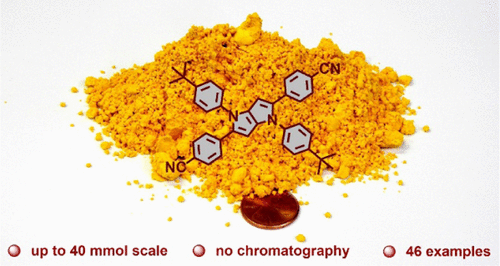
A thorough investigation has enabled the optimization of the synthesis of 1,4-dihydro-pyrrolo[3,2-b]pyrroles. Although salts of such metals as vanadium, niobium, cerium, and manganese were found to facilitate the formation of 1,4-dihydro-pyrrolo[3,2-b]pyrroles from amines, aldehydes, and diacetyl, we confirmed that iron salts are the most efficient catalysts. The conditions identified (first step: toluene/AcOH = 1:1, 1 h, 50 °C; second step: toluene/AcOH = 1:1, Fe(ClO4)3·H2O, 16 h, 50 °C) resulted in the formation of tetraarylpyrrolo[3,2-b]pyrroles in a 6–69% yield. For the first time, very electron-rich substituents (4-Me2NC6H4, 3-(OH)C6H4, pyrrol-2-yl) originating from aldehydes and sterically hindered substituents (2-ClC6H4, 2-BrC6H4, 2-CNC6H4, 2-(CO2Me)C6H4, 2-(TMS-C≡C)C6H4) present on anilines can be appended to the pyrrolo[3,2-b]pyrrole core. It is now also possible to prepare 1,4-dihydropyrrolo[3,2-b]pyrroles bearing an ordered arrangement of N-substituents and C-substituents ranging from coumarin, quinoline, phthalimide to truxene. These advances in scope enable independent regulations of many desired photophysical properties, including the Stokes shift value and emission color ranging from violet-blue through deep blue, green, yellow to red. Simultaneously, the optimized conditions have finally allowed the synthesis of these extremely promising heterocycles in amounts of more than 10 g per run without a concomitant decrease in yield or product contamination. Empowered with better functional group compatibility, novel derivatization strategies were developed.
中文翻译:

大规模合成多功能1,4-二氢-吡咯并[3,2-b]吡咯的方法。
彻底的研究已使1,4-二氢-吡咯并[3,2- b ]吡咯的合成最优化。尽管发现钒,铌,铈和锰等金属的盐可促进由胺,醛和二乙酰基形成1,4-二氢-吡咯并[3,2- b ]吡咯,但我们证实铁盐是最有效的催化剂。确定的条件(第一步:甲苯/ AcOH = 1:1,1 h,50°C;第二步:甲苯/ AcOH = 1:1,Fe(ClO 4)3 ·H 2 O,16 h,50°C )导致四芳基吡咯并[3,2- b ]吡咯的形成,产率为6-69%。首次出现非常富电子的取代基(4-Me 2 NC 6 H4,3-(OH)C 6 H ^ 4,吡咯-2-基)由醛始发和空间位阻的取代基(2-CLC 6 ħ 4,2- BRC 6 ħ 4,2-CNC 6 ħ 4,2-( CO 2我)C 6 H ^ 4,2-(TMS-C≡C)C 6 H ^ 4)上苯胺本可以被附加到所述吡咯并[3,2- b ]吡咯芯。现在也可以制备带有N-取代基和C的有序排列的1,4-二氢吡咯并[3,2- b ]吡咯-取代基,包括香豆素,喹啉,邻苯二甲酰亚胺和丁三烯。这些范围的进步使得能够对许多所需的光物理特性进行独立调节,包括斯托克斯位移值和发射颜色,范围从紫蓝色到深蓝色,绿色,黄色到红色。同时,最优化的条件最终使这些极有希望的杂环的合成量超过每次运行10 g,而收率或产品污染却没有随之下降。具有更好的官能团兼容性,开发了新颖的衍生化策略。
更新日期:2020-11-06
中文翻译:

大规模合成多功能1,4-二氢-吡咯并[3,2-b]吡咯的方法。
彻底的研究已使1,4-二氢-吡咯并[3,2- b ]吡咯的合成最优化。尽管发现钒,铌,铈和锰等金属的盐可促进由胺,醛和二乙酰基形成1,4-二氢-吡咯并[3,2- b ]吡咯,但我们证实铁盐是最有效的催化剂。确定的条件(第一步:甲苯/ AcOH = 1:1,1 h,50°C;第二步:甲苯/ AcOH = 1:1,Fe(ClO 4)3 ·H 2 O,16 h,50°C )导致四芳基吡咯并[3,2- b ]吡咯的形成,产率为6-69%。首次出现非常富电子的取代基(4-Me 2 NC 6 H4,3-(OH)C 6 H ^ 4,吡咯-2-基)由醛始发和空间位阻的取代基(2-CLC 6 ħ 4,2- BRC 6 ħ 4,2-CNC 6 ħ 4,2-( CO 2我)C 6 H ^ 4,2-(TMS-C≡C)C 6 H ^ 4)上苯胺本可以被附加到所述吡咯并[3,2- b ]吡咯芯。现在也可以制备带有N-取代基和C的有序排列的1,4-二氢吡咯并[3,2- b ]吡咯-取代基,包括香豆素,喹啉,邻苯二甲酰亚胺和丁三烯。这些范围的进步使得能够对许多所需的光物理特性进行独立调节,包括斯托克斯位移值和发射颜色,范围从紫蓝色到深蓝色,绿色,黄色到红色。同时,最优化的条件最终使这些极有希望的杂环的合成量超过每次运行10 g,而收率或产品污染却没有随之下降。具有更好的官能团兼容性,开发了新颖的衍生化策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号