当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Metal-free Allylic C-H Amination of Terpenoids
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-09-10 , DOI: 10.1021/jacs.0c06997 Wei Pin Teh 1 , Derek C Obenschain 1 , Blaise M Black 1 , Forrest E Michael 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-09-10 , DOI: 10.1021/jacs.0c06997 Wei Pin Teh 1 , Derek C Obenschain 1 , Blaise M Black 1 , Forrest E Michael 1
Affiliation
|
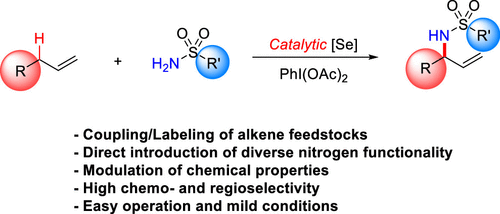
The selective replacement of C-H bonds in complex molecules, especially natural products like terpenoids, is a highly efficient way to introduce new functionality and/or couple fragments. Here, we report the development of a new metal-free allylic amination of alkenes that allows the introduction of a wide range of nitrogen functionality at the allylic position of alkenes with unique regioselectivity and no allylic transposition. This reaction employs catalytic amounts of selenium in the form of phosphine selenides or selenoureas. Simple sulfonamides and sulfamates can be used directly in the reaction without the need to prepare isolated nitrenoid precursors. We demonstrate the utility of this transformation by aminating a large set of terpenoids in high yield and regioselectivity.
中文翻译:

萜类化合物的催化无金属烯丙基 CH 胺化
选择性替换复杂分子中的 CH 键,尤其是萜类化合物等天然产物,是引入新功能和/或偶联片段的高效方法。在这里,我们报告了一种新的烯烃无金属烯丙基胺化的开发,它允许在烯烃的烯丙基位置引入广泛的氮官能团,具有独特的区域选择性和无烯丙基转座。该反应使用催化量的硒化膦或硒脲形式的硒。简单的磺酰胺和氨基磺酸盐可直接用于反应,无需制备分离的类氮烯前体。我们通过以高产率和区域选择性胺化大量萜类化合物来证明这种转化的效用。
更新日期:2020-09-10
中文翻译:

萜类化合物的催化无金属烯丙基 CH 胺化
选择性替换复杂分子中的 CH 键,尤其是萜类化合物等天然产物,是引入新功能和/或偶联片段的高效方法。在这里,我们报告了一种新的烯烃无金属烯丙基胺化的开发,它允许在烯烃的烯丙基位置引入广泛的氮官能团,具有独特的区域选择性和无烯丙基转座。该反应使用催化量的硒化膦或硒脲形式的硒。简单的磺酰胺和氨基磺酸盐可直接用于反应,无需制备分离的类氮烯前体。我们通过以高产率和区域选择性胺化大量萜类化合物来证明这种转化的效用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号