当前位置:
X-MOL 学术
›
ACS Appl. Bio Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characteristics of Molecularly Engineered Anticancer Drug Conjugated Organic Nanomicelles for Site-Selective Cancer Cell Rupture and Growth Inhibition of Tumor Spheroids
ACS Applied Bio Materials ( IF 4.6 ) Pub Date : 2020-09-09 , DOI: 10.1021/acsabm.0c00913 Nishant Kumar Jain 1 , Shalini Dimri 2, 3 , Rajendra Prasad 1 , Gayathri Ravichandran 2, 3 , Vegi Naidu 4 , Abhijit De 2, 3 , Rohit Srivastava 1
ACS Applied Bio Materials ( IF 4.6 ) Pub Date : 2020-09-09 , DOI: 10.1021/acsabm.0c00913 Nishant Kumar Jain 1 , Shalini Dimri 2, 3 , Rajendra Prasad 1 , Gayathri Ravichandran 2, 3 , Vegi Naidu 4 , Abhijit De 2, 3 , Rohit Srivastava 1
Affiliation
|
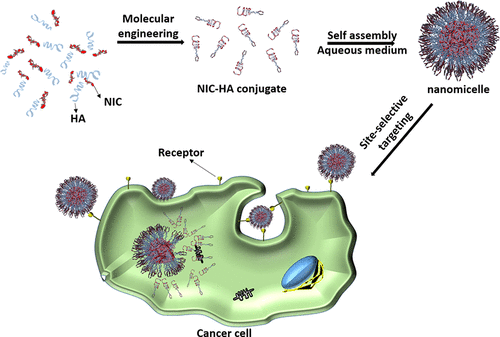
Site-selective uptake and specific biodistribution of chemotherapeutic drugs are essential prerequisites for targeted cancer therapy. Especially, antibody and peptide conjugated drugs have been attempted as localized therapeutic agents. However, the characteristics of drug conjugated nanosystems are less explored, which are limited with their toxicity, low therapeutic efficacy, complicated synthesis, and high costs. Herein, we report a biocompatible (about 95%) molecularly engineered anticancer drug conjugated nanomicelles (∼200 nm in size) for site-selective CD44 overexpressed cancer cell rupture and tumor growth inhibition. Microscopic analysis demonstrates the distinct visualization of organic–organic interfaces (∼5 nm), which are corroborated with spectroscopic measurements confirmed the conjugation of niclosamide drug with hyaluronic acid (NIC-HA). Uniformly distributed hemocompatible (about 99%) organic nanomicelles exhibit the cellular membrane and cytoplasmic targeting with significant cellular rupture (IC50 of 4 μM for MDA MB 231 cells) indicating their inherent targeting ability for cancer cells and cancer stem cells. An inclusive in vitro and in vivo analysis for targeted antitumor activity (HT1080 tumor xenograft model) of NIC-HA nanoconjugates (∼24.6% loading) exhibited promising cancer cell death and tumor growth inhibition (60%, p < 0.05) due to STAT-3 signaling pathway inhibition and induction of apoptosis in CD44-positive triple negative breast cancer cells.
中文翻译:

分子工程抗癌药物偶联有机纳米胶束用于癌细胞定点破裂和肿瘤球体生长抑制的特性
化疗药物的位点选择性摄取和特异性生物分布是靶向癌症治疗的必要先决条件。特别是,抗体和肽缀合的药物已被尝试作为局部治疗剂。然而,药物共轭纳米系统的特点研究较少,受限于其毒性、疗效低、合成复杂和成本高等特点。在此,我们报告了一种生物相容性(约 95%)分子工程抗癌药物偶联纳米胶束(尺寸约为 200 nm),用于位点选择性 CD44 过表达的癌细胞破裂和肿瘤生长抑制。显微分析证明了有机-有机界面的明显可视化(~5 nm),光谱测量证实了氯硝柳胺药物与透明质酸(NIC-HA)的结合。均匀分布的血液相容性(约 99%)有机纳米胶束表现出细胞膜和细胞质靶向性,具有显着的细胞破裂(ICMDA MB 231 细胞的 4 μM 中的50个)表明它们对癌细胞和癌症干细胞的固有靶向能力。对 NIC-HA 纳米缀合物(约 24.6% 负载)的靶向抗肿瘤活性(HT1080 肿瘤异种移植模型)的包容性体外和体内分析显示出有希望的癌细胞死亡和肿瘤生长抑制(60%,p < 0.05)由于 STAT- 3信号通路抑制和诱导CD44阳性三阴性乳腺癌细胞凋亡。
更新日期:2020-10-21
中文翻译:

分子工程抗癌药物偶联有机纳米胶束用于癌细胞定点破裂和肿瘤球体生长抑制的特性
化疗药物的位点选择性摄取和特异性生物分布是靶向癌症治疗的必要先决条件。特别是,抗体和肽缀合的药物已被尝试作为局部治疗剂。然而,药物共轭纳米系统的特点研究较少,受限于其毒性、疗效低、合成复杂和成本高等特点。在此,我们报告了一种生物相容性(约 95%)分子工程抗癌药物偶联纳米胶束(尺寸约为 200 nm),用于位点选择性 CD44 过表达的癌细胞破裂和肿瘤生长抑制。显微分析证明了有机-有机界面的明显可视化(~5 nm),光谱测量证实了氯硝柳胺药物与透明质酸(NIC-HA)的结合。均匀分布的血液相容性(约 99%)有机纳米胶束表现出细胞膜和细胞质靶向性,具有显着的细胞破裂(ICMDA MB 231 细胞的 4 μM 中的50个)表明它们对癌细胞和癌症干细胞的固有靶向能力。对 NIC-HA 纳米缀合物(约 24.6% 负载)的靶向抗肿瘤活性(HT1080 肿瘤异种移植模型)的包容性体外和体内分析显示出有希望的癌细胞死亡和肿瘤生长抑制(60%,p < 0.05)由于 STAT- 3信号通路抑制和诱导CD44阳性三阴性乳腺癌细胞凋亡。











































 京公网安备 11010802027423号
京公网安备 11010802027423号