当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engaging thieno[2,3‐b]indole‐2,3‐dione for the efficient synthesis of spiro[indoline‐3,4′‐thiopyrano[2,3‐b]indole] by reaction with N‐substituted isatilidenes
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-09-10 , DOI: 10.1002/jhet.4147 Noble V. Thomas 1 , Vidya Sathi 1 , Ani Deepthi 1 , Sruthi Leena 1 , Sidharth Chopra 2
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-09-10 , DOI: 10.1002/jhet.4147 Noble V. Thomas 1 , Vidya Sathi 1 , Ani Deepthi 1 , Sruthi Leena 1 , Sidharth Chopra 2
Affiliation
|
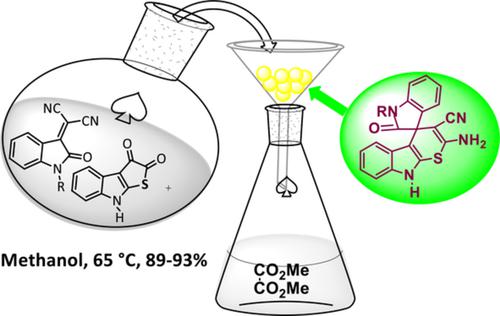
A simple and efficient method, proceeding through a new mechanistic pathway, for the synthesis of spiro[indoline‐3,4‐thiopyrano[2.3‐b]indole derivatives have been developed by exploiting the reaction of thieno[2,3‐b]indole‐2,3‐dione with N‐substituted isatilidenes. The compounds synthesized have been screened for antibacterial activity. The generality of the reaction and mechanistic rationale are presented.
中文翻译:

噻吩[2,3-b]吲哚-2,3-二酮与N-取代的异戊二烯反应,可有效合成螺[吲哚啉-3,4'-硫代吡喃并[2,3-b]吲哚]
通过利用噻吩并[2,3-b]吲哚的反应,开发了一种简单有效的方法,通过新的机理途径合成螺[吲哚啉-3,4-硫代吡喃并[2.3-b]吲哚衍生物-2,3-二酮与N-取代的异戊二烯。已经筛选了合成的化合物的抗菌活性。介绍了反应的一般性和机理原理。
更新日期:2020-09-10
中文翻译:

噻吩[2,3-b]吲哚-2,3-二酮与N-取代的异戊二烯反应,可有效合成螺[吲哚啉-3,4'-硫代吡喃并[2,3-b]吲哚]
通过利用噻吩并[2,3-b]吲哚的反应,开发了一种简单有效的方法,通过新的机理途径合成螺[吲哚啉-3,4-硫代吡喃并[2.3-b]吲哚衍生物-2,3-二酮与N-取代的异戊二烯。已经筛选了合成的化合物的抗菌活性。介绍了反应的一般性和机理原理。









































 京公网安备 11010802027423号
京公网安备 11010802027423号