当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sustainable Synthetic Approaches for 3‐Aminoimidazo‐fused Heterocycles via Groebke‐Blackburn‐Bienaymé Process
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-09-10 , DOI: 10.1002/slct.202002894 Sivagami Mathavan 1 , Rajesh B. R. D. Yamajala 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-09-10 , DOI: 10.1002/slct.202002894 Sivagami Mathavan 1 , Rajesh B. R. D. Yamajala 1
Affiliation
|
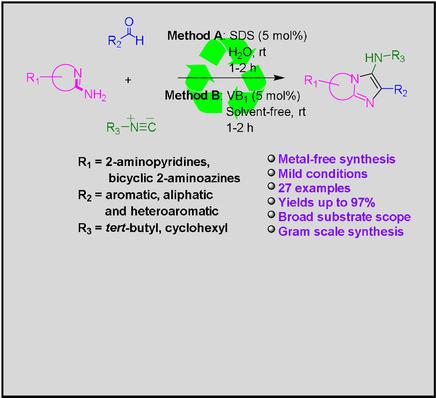
Two different sustainable and efficient synthetic methods have been developed for the synthesis of a variety of 3‐aminoimidazo fused heterocycles by one‐pot three component reaction of 2‐aminoazines, aldehydes and isocyanides. The methods adopted were (a) aqueous micellar mediated condition (method A) (b) VB1 catalyzed reaction under solvent‐free condition (method B). Excellent yields were obtained for a broad range of biologically significant imidazopyridine core molecules under green conditions at room temperature. Synthesis in aqueous micellar medium, mild reaction conditions, organocatalyst, easy work‐up and isolation, and gram scale synthesis, are some of the advantages of these protocols.
中文翻译:

通过Groebke-Blackburn-Bienaymé过程进行3-氨基咪唑并合杂环的可持续合成方法
通过2-氨基嗪,醛和异氰酸酯的一锅三组分反应,已开发出两种不同的可持续有效合成方法来合成各种3-氨基咪唑稠合的杂环。采用的方法是(a)胶束介导的水性条件(方法A)(b)在无溶剂条件下VB 1催化的反应(方法B)。在室温下绿色条件下,对于具有广泛生物学意义的咪唑并吡啶核心分子,均获得了优异的收率。这些规程的优点是在水性胶束介质中进行合成,温和的反应条件,有机催化剂,易于处理和分离以及克规模的合成。
更新日期:2020-09-10
中文翻译:

通过Groebke-Blackburn-Bienaymé过程进行3-氨基咪唑并合杂环的可持续合成方法
通过2-氨基嗪,醛和异氰酸酯的一锅三组分反应,已开发出两种不同的可持续有效合成方法来合成各种3-氨基咪唑稠合的杂环。采用的方法是(a)胶束介导的水性条件(方法A)(b)在无溶剂条件下VB 1催化的反应(方法B)。在室温下绿色条件下,对于具有广泛生物学意义的咪唑并吡啶核心分子,均获得了优异的收率。这些规程的优点是在水性胶束介质中进行合成,温和的反应条件,有机催化剂,易于处理和分离以及克规模的合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号