当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bonding Energetics of Palladium-amido/aryloxide Complexes in DMSO: Implications for Pd-mediated Aniline Activation.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-09-10 , DOI: 10.1002/anie.202011313 Jun-Yan Wu 1 , Zhen Li 1 , Jin-Dong Yang 1 , Jin-Pei Cheng 1, 2
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-09-10 , DOI: 10.1002/anie.202011313 Jun-Yan Wu 1 , Zhen Li 1 , Jin-Dong Yang 1 , Jin-Pei Cheng 1, 2
Affiliation
|
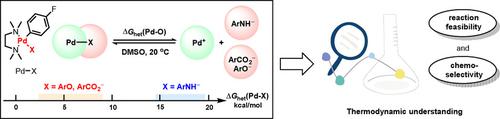
Thermodynamic knowledge of the metal–ligand (M−L) σ‐bond strength is crucial to understanding metal‐mediated transformations. Here, we developed a method for determining the Pd−X (X=OR and NHAr) bond heterolysis energies (ΔGhet(Pd−X)) in DMSO taking [(tmeda)PdArX] (tmeda=N,N,N′,N′‐tetramethylethylenediamine) as the model complexes. The ΔGhet(Pd−X) scales span a range of 2.6–9.0 kcal mol−1 for ΔGhet(Pd−O) values and of 14.5–19.5 kcal mol−1 for ΔGhet(Pd−N) values, respectively, implying a facile heterolytic detachment of the Pd ligands. Structure‐reactivity analyses of a modeling Pd‐mediated X−H bond activation reveal that the M−X bond metathesis is dominated by differences of the X−H and Pd−X bond strengths, the former being more influential. The ΔGhet(Pd−X) and pKa(X−H) parameters enable regulation of reaction thermodynamics and chemoselectivity and diagnosing the probability of aniline activation with Pd−X complexes.
中文翻译:

在DMSO中钯-酰胺基/芳基氧化物络合物的键能:对Pd介导的苯胺活化的影响。
金属-配体(ML)σ-键强度的热力学知识对于理解金属介导的转变至关重要。在这里,我们[[tmeda] PdArX](tmeda = N,N,N ' )提出了一种确定DMSO中Pd-X(X = OR和NHAr)键杂合能(ΔG het(Pd-X))的方法。,N'-四甲基乙二胺)作为模型络合物。该Δ ģ HET(PD-X)秤跨越范围2.6-9.0千卡摩尔-1为Δ G ^ HET(PD-O)的值和14.5-19.5千卡摩尔-1为Δ G ^ HET(Pd-N)值分别表示Pd配体的容易杂化分离。建模的Pd介导的X-H键活化的结构反应性分析表明,MX键的复分解作用主要受X-H和Pd-X键强度的差异影响,前者的影响更大。该Δ ģ HET(PD-X)和p ķ一个(X-H)参数使反应热力学和化学选择性的调节和诊断苯胺活化用Pd-X配合物的概率。
更新日期:2020-09-10
中文翻译:

在DMSO中钯-酰胺基/芳基氧化物络合物的键能:对Pd介导的苯胺活化的影响。
金属-配体(ML)σ-键强度的热力学知识对于理解金属介导的转变至关重要。在这里,我们[[tmeda] PdArX](tmeda = N,N,N ' )提出了一种确定DMSO中Pd-X(X = OR和NHAr)键杂合能(ΔG het(Pd-X))的方法。,N'-四甲基乙二胺)作为模型络合物。该Δ ģ HET(PD-X)秤跨越范围2.6-9.0千卡摩尔-1为Δ G ^ HET(PD-O)的值和14.5-19.5千卡摩尔-1为Δ G ^ HET(Pd-N)值分别表示Pd配体的容易杂化分离。建模的Pd介导的X-H键活化的结构反应性分析表明,MX键的复分解作用主要受X-H和Pd-X键强度的差异影响,前者的影响更大。该Δ ģ HET(PD-X)和p ķ一个(X-H)参数使反应热力学和化学选择性的调节和诊断苯胺活化用Pd-X配合物的概率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号