当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
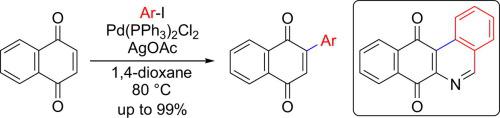
Palladium-catalyzed arylation of 1,4-naphthoquinones with aryl iodides and its synthetic application to the benzo[b]phenanthridine skeleton
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2020-09-10 , DOI: 10.1016/j.tetlet.2020.152446 Yusuke Akagi , Toshiya Komatsu
中文翻译:

钯催化的1,4-萘醌与芳基碘化物的芳基化及其在苯并[ b ]菲啶骨架上的合成应用
更新日期:2020-10-12
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2020-09-10 , DOI: 10.1016/j.tetlet.2020.152446 Yusuke Akagi , Toshiya Komatsu
|
We report a Pd-catalyzed arylation of 1,4-naphthoquinones with aryl iodides. This reaction shows excellent functional group tolerance and high regioselectivity when using nonsymmetric 1,4-naphthoquinone. Furthermore, the resulting 2-aryl-1,4-naphthoquinone could be successfully converted into benzo[b]phenanthridine-7,12-dione through treatment with aqueous ammonia, followed by oxidative cyclization using MnO2.
中文翻译:

钯催化的1,4-萘醌与芳基碘化物的芳基化及其在苯并[ b ]菲啶骨架上的合成应用
我们报告钯与芳基碘化物催化的芳基化的芳基化。当使用非对称的1,4-萘醌时,该反应显示出优异的官能团耐受性和高的区域选择性。此外,通过用氨水处理,然后使用MnO 2进行氧化环化,可以将所得的2-芳基-1,4-萘醌成功地转化为苯并[ b ]菲啶-7,12-二酮。



























 京公网安备 11010802027423号
京公网安备 11010802027423号