当前位置:
X-MOL 学术
›
Process Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enzymatic synthesis of N-10-undecenoyl-phenylalanine catalysed by aminoacylases from Streptomyces ambofaciens
Process Biochemistry ( IF 3.7 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.procbio.2020.09.009 Mohamed Chafik Bourkaib , Stephane Delaunay , Xavier Framboisier , Catherine Humeau , Jérôme Guilbot , Cecile Bize , Estelle Illous , Isabelle Chevalot , Yann Guiavarc’h
Process Biochemistry ( IF 3.7 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.procbio.2020.09.009 Mohamed Chafik Bourkaib , Stephane Delaunay , Xavier Framboisier , Catherine Humeau , Jérôme Guilbot , Cecile Bize , Estelle Illous , Isabelle Chevalot , Yann Guiavarc’h
|
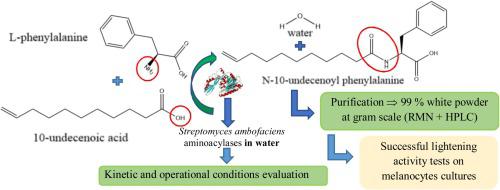
Abstract Due to its physico-chemical and biological activities, N-10-undecenoyl-phenylalanine (C11’F) is one of the most interesting lipoaminoacids used in cosmetic and pharmaceutical industries. Its production is currently based on the Schotten-Baumann chemical reaction, which shows some environmental issues in terms of effluents. As a possible biocatalytic alternative, this study presents the evaluation of the reactional and process conditions allowing the production of C11’F using aminoacylases from Streptomyces ambofaciens culture. These aminoacylases showed the best activity at 45 °C and pH between 7 and 8 with a moderate thermal stability. The influence of substrates concentrations on the kinetic parameters of C11’F synthesis showed a more important impact of the phenylalanine concentration as compared to the 10-undecenoic acid concentration. As a reactional product, C11’F appeared to have an inhibitory effect on the enzymatic N-acylation. Cobalt addition allowed an eleven-fold increase of the reaction rate. Batch reactors were used with free aminoacylases without impact on the final C11’F concentration. For the first time, enzymatically produced C11’F was finally purified at the gram scale as a 99% purity white powder. The evaluation of the biological activity on melanocytes cultures showed the presence of skin lightening activity similar to the one obtained with the chemically produced C11’F.
中文翻译:

来自 Streptomyces ambofaciens 的氨基酰化酶催化 N-10-十一烯酰基-苯丙氨酸的酶促合成
摘要 由于其物理化学和生物活性,N-10-十一碳烯酰苯丙氨酸 (C11'F) 是化妆品和制药行业中最有趣的脂族氨基酸之一。它的生产目前基于 Schotten-Baumann 化学反应,这表明在流出物方面存在一些环境问题。作为一种可能的生物催化替代方案,本研究评估了允许使用来自 Streptomyces ambofaciens 培养物的氨酰酶生产 C11'F 的反应和工艺条件。这些氨基酰化酶在 45°C 和 pH 值介于 7 和 8 之间时表现出最佳活性,具有中等热稳定性。底物浓度对 C11'F 合成动力学参数的影响表明,与 10-十一碳烯酸浓度相比,苯丙氨酸浓度的影响更为重要。作为反应产物,C11'F 似乎对酶促 N-酰化具有抑制作用。添加钴使反应速率提高了 11 倍。间歇反应器与游离氨基酰化酶一起使用,对最终的 C11'F 浓度没有影响。第一次,酶促生产的 C11'F 最终以克级规模纯化为 99% 纯度的白色粉末。对黑素细胞培养物的生物活性的评估表明存在类似于用化学产生的 C11'F 获得的亮肤活性。酶促产生的 C11'F 最终在克级纯化为 99% 纯度的白色粉末。对黑素细胞培养物的生物活性的评估表明存在类似于用化学产生的 C11'F 获得的亮肤活性。酶促产生的 C11'F 最终在克级纯化为 99% 纯度的白色粉末。对黑素细胞培养物的生物活性的评估表明存在类似于用化学产生的 C11'F 获得的亮肤活性。
更新日期:2020-12-01
中文翻译:

来自 Streptomyces ambofaciens 的氨基酰化酶催化 N-10-十一烯酰基-苯丙氨酸的酶促合成
摘要 由于其物理化学和生物活性,N-10-十一碳烯酰苯丙氨酸 (C11'F) 是化妆品和制药行业中最有趣的脂族氨基酸之一。它的生产目前基于 Schotten-Baumann 化学反应,这表明在流出物方面存在一些环境问题。作为一种可能的生物催化替代方案,本研究评估了允许使用来自 Streptomyces ambofaciens 培养物的氨酰酶生产 C11'F 的反应和工艺条件。这些氨基酰化酶在 45°C 和 pH 值介于 7 和 8 之间时表现出最佳活性,具有中等热稳定性。底物浓度对 C11'F 合成动力学参数的影响表明,与 10-十一碳烯酸浓度相比,苯丙氨酸浓度的影响更为重要。作为反应产物,C11'F 似乎对酶促 N-酰化具有抑制作用。添加钴使反应速率提高了 11 倍。间歇反应器与游离氨基酰化酶一起使用,对最终的 C11'F 浓度没有影响。第一次,酶促生产的 C11'F 最终以克级规模纯化为 99% 纯度的白色粉末。对黑素细胞培养物的生物活性的评估表明存在类似于用化学产生的 C11'F 获得的亮肤活性。酶促产生的 C11'F 最终在克级纯化为 99% 纯度的白色粉末。对黑素细胞培养物的生物活性的评估表明存在类似于用化学产生的 C11'F 获得的亮肤活性。酶促产生的 C11'F 最终在克级纯化为 99% 纯度的白色粉末。对黑素细胞培养物的生物活性的评估表明存在类似于用化学产生的 C11'F 获得的亮肤活性。







































 京公网安备 11010802027423号
京公网安备 11010802027423号