Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2020-09-10 , DOI: 10.1016/j.bmcl.2020.127544 Lalitha Gummidi 1 , Nagaraju Kerru 1 , Paul Awolade 1 , Asif Raza 2 , Arun K Sharma 2 , Parvesh Singh 1
|
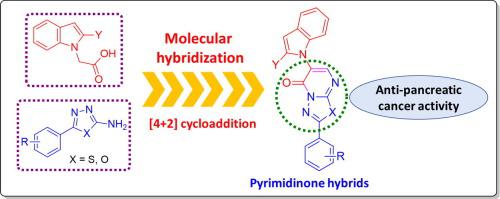
New indole-tethered [1,3,4]thiadiazolo[3,2-a]pyrimidin-5-one (8a-j) and [1,3,4]oxadiazolo[3,2-a]pyrimidin-5-one hybrids (9a-e) were synthesized using [4+2] cycloaddition reactions of functionalized 1,3-diazabuta-1,3-dienes with indole-ketenes. All molecular hybrids were structurally characterized by spectroscopic techniques (IR, NMR, and HRMS) and screened for their anti-pancreatic cancer activity in vitro. The [1,3,4]oxadiazolo[3,2-a]pyrimidin-5-one hybrids (9a-e) showed stronger anti-pancreatic cancer activity than the [1,3,4]thiadiazolo[3,2-a]pyrimidin-5-one hybrids (8a-j) against the PANC-1 cell line. Compound 9d bearing an ortho-chlorophenyl moiety emerged as the most potent anti-pancreatic cancer agent with an IC50 value of 7.7 ± 0.4 µM, much superior to the standard drug Gemcitabine (IC50 > 500 µM). The discovery of these [1,3,4]thiadiazolo and [1,3,4]oxadiazolo[3,2-a]pyrimidin-5-one hybrids elicits their potentials as pursuable candidates for pancreatic cancer chemotherapy.
中文翻译:

吲哚系[1,3,4]噻二唑和[1,3,4]恶二唑并[3,2-a]嘧啶-5-酮杂合体的合成作为抗胰腺癌药物。
新的吲哚系[1,3,4]噻二唑[3,2 - a ]嘧啶-5-酮(8a - j)和[1,3,4]恶二唑[3,2 - a ]嘧啶-5-酮杂种(9a-e)使用功能化的1,3-二氮杂丁-1,3-二烯与吲哚-乙烯酮的[4 + 2]环加成反应合成。所有分子杂种均通过光谱技术(IR,NMR和HRMS)进行结构表征,并筛选其体外抗胰腺癌活性。[1,3,4]恶二唑[3,2 - a ]嘧啶-5-酮杂种(9a-e)显示出比[1,3,4]噻二唑[3,2- a ]更强的抗胰腺癌活性。] pyrimidin-5-one杂种(8a-j)针对PANC-1细胞系。带有邻氯苯基部分的化合物9d以最强效的抗胰腺癌药物出现,其IC 50值为7.7±0.4 µM,大大优于标准药物吉西他滨(IC 50 > 500 µM)。这些[1,3,4]噻二唑和[1,3,4]恶二唑[3,2 - a ]嘧啶-5-酮杂化物的发现激发了它们作为胰腺癌化学疗法的潜在候选者的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号