当前位置:
X-MOL 学术
›
RSC Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Met80 and Tyr67 affect the chemical unfolding of yeast cytochrome c: comparing the solution vs. immobilized state
RSC Chemical Biology ( IF 4.2 ) Pub Date : 2020-09-09 , DOI: 10.1039/d0cb00115e Alessandro Paradisi 1, 2, 3, 4 , Lidia Lancellotti 5, 6, 7, 8 , Marco Borsari 5, 6, 7, 8 , Marzia Bellei 6, 7, 8, 9 , Carlo Augusto Bortolotti 6, 7, 8, 9 , Giulia Di Rocco 6, 7, 8, 9 , Antonio Ranieri 6, 7, 8, 9 , Marco Sola 6, 7, 8, 9 , Gianantonio Battistuzzi 5, 6, 7, 8
RSC Chemical Biology ( IF 4.2 ) Pub Date : 2020-09-09 , DOI: 10.1039/d0cb00115e Alessandro Paradisi 1, 2, 3, 4 , Lidia Lancellotti 5, 6, 7, 8 , Marco Borsari 5, 6, 7, 8 , Marzia Bellei 6, 7, 8, 9 , Carlo Augusto Bortolotti 6, 7, 8, 9 , Giulia Di Rocco 6, 7, 8, 9 , Antonio Ranieri 6, 7, 8, 9 , Marco Sola 6, 7, 8, 9 , Gianantonio Battistuzzi 5, 6, 7, 8
Affiliation
|
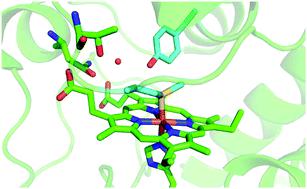
Urea-induced denaturation of the Met80Ala and Met80Ala/Tyr67Ala variants of S. cerevisiae iso-1 cytochrome c (ycc) was studied through variable temperature diffusive cyclic voltammetry and electronic absorption, CD and MCD spectroscopies. The susceptibility to unfolding of both variants – represented by the free energy of unfolding at denaturant infinite dilution, 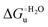 – is greater compared to the species showing an intact Met/His coordination, as observed previously for the same species immobilized onto a functionalized electrode. This is consistent with the role of the axial Fe–(S)Met bond and the H-bond network involving Tyr67 in stabilizing the polypeptide matrix in the heme crevice. Notably, we find that the unfolding propensity and axial heme iron coordination of the present Fe–(S)Met bond-deprived variants are affected by the motional regime of the protein. In particular, electrostatic adsorption onto a negatively charged SAM surface – which would mimic the phospholipidic inner mitochondrial membrane – facilitates unfolding compared to the solution state, especially at room temperature. This finding has physiological relevance related to the cytochrome c interaction with cardiolipin at the IMM in the early stages of apoptosis. Moreover, while both immobilized variants maintain the His/OH− axial heme iron coordination up to 7 M urea, the same species in solution are subjected to urea-induced replacement of the axial hydroxide ligand by a His ligand. The contributions of the enthalpic and entropic terms to
– is greater compared to the species showing an intact Met/His coordination, as observed previously for the same species immobilized onto a functionalized electrode. This is consistent with the role of the axial Fe–(S)Met bond and the H-bond network involving Tyr67 in stabilizing the polypeptide matrix in the heme crevice. Notably, we find that the unfolding propensity and axial heme iron coordination of the present Fe–(S)Met bond-deprived variants are affected by the motional regime of the protein. In particular, electrostatic adsorption onto a negatively charged SAM surface – which would mimic the phospholipidic inner mitochondrial membrane – facilitates unfolding compared to the solution state, especially at room temperature. This finding has physiological relevance related to the cytochrome c interaction with cardiolipin at the IMM in the early stages of apoptosis. Moreover, while both immobilized variants maintain the His/OH− axial heme iron coordination up to 7 M urea, the same species in solution are subjected to urea-induced replacement of the axial hydroxide ligand by a His ligand. The contributions of the enthalpic and entropic terms to 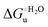 were found to be opposite (H–S compensation), indicating that the unfolding thermodynamics are strongly affected by changes in the hydrogen bonding network in the hydration sphere of the protein.
were found to be opposite (H–S compensation), indicating that the unfolding thermodynamics are strongly affected by changes in the hydrogen bonding network in the hydration sphere of the protein.
中文翻译:

Met80和Tyr67影响酵母细胞色素c的化学解折叠:比较溶液与固定状态
通过可变温度扩散循环伏安法和电子吸收,CD和MCD光谱研究了尿素诱导的酿酒酵母iso-1细胞色素c(ycc)Met80Ala和Met80Ala / Tyr67Ala变体的变性。两种变体都易于展开-以变性剂无限稀释时展开的自由能表示,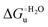 –与显示完整的Met / His配比的物种相比更大,如先前观察到的固定在功能化电极上的相同物种。这与轴向Fe–(S)Met键和涉及Tyr67的H键网络在稳定血红素缝隙中的多肽基质中的作用是一致的。值得注意的是,我们发现当前Fe–(S)Met键缺失的变异体的展开倾向和轴向血红素铁配位受蛋白质的运动方式影响。特别是,与溶液状态相比,静电吸附到带负电荷的SAM表面上(会模拟磷脂质内线粒体膜)有助于展开,特别是在室温下。这一发现具有与细胞色素c相关的生理相关性在细胞凋亡早期与IMM的心磷脂相互作用。此外,虽然两个固定的变体保持他的/ OH -轴向血红素铁协调高达7M尿素,溶液中的相同物种经受尿素诱导的替换由他的配位体的轴向氢氧化物配位体。
–与显示完整的Met / His配比的物种相比更大,如先前观察到的固定在功能化电极上的相同物种。这与轴向Fe–(S)Met键和涉及Tyr67的H键网络在稳定血红素缝隙中的多肽基质中的作用是一致的。值得注意的是,我们发现当前Fe–(S)Met键缺失的变异体的展开倾向和轴向血红素铁配位受蛋白质的运动方式影响。特别是,与溶液状态相比,静电吸附到带负电荷的SAM表面上(会模拟磷脂质内线粒体膜)有助于展开,特别是在室温下。这一发现具有与细胞色素c相关的生理相关性在细胞凋亡早期与IMM的心磷脂相互作用。此外,虽然两个固定的变体保持他的/ OH -轴向血红素铁协调高达7M尿素,溶液中的相同物种经受尿素诱导的替换由他的配位体的轴向氢氧化物配位体。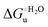 发现焓和熵项的贡献是相反的(H–S补偿),表明展开的热力学受到蛋白质水化范围内氢键网络变化的强烈影响。
发现焓和熵项的贡献是相反的(H–S补偿),表明展开的热力学受到蛋白质水化范围内氢键网络变化的强烈影响。
更新日期:2020-11-03
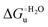 – is greater compared to the species showing an intact Met/His coordination, as observed previously for the same species immobilized onto a functionalized electrode. This is consistent with the role of the axial Fe–(S)Met bond and the H-bond network involving Tyr67 in stabilizing the polypeptide matrix in the heme crevice. Notably, we find that the unfolding propensity and axial heme iron coordination of the present Fe–(S)Met bond-deprived variants are affected by the motional regime of the protein. In particular, electrostatic adsorption onto a negatively charged SAM surface – which would mimic the phospholipidic inner mitochondrial membrane – facilitates unfolding compared to the solution state, especially at room temperature. This finding has physiological relevance related to the cytochrome c interaction with cardiolipin at the IMM in the early stages of apoptosis. Moreover, while both immobilized variants maintain the His/OH− axial heme iron coordination up to 7 M urea, the same species in solution are subjected to urea-induced replacement of the axial hydroxide ligand by a His ligand. The contributions of the enthalpic and entropic terms to
– is greater compared to the species showing an intact Met/His coordination, as observed previously for the same species immobilized onto a functionalized electrode. This is consistent with the role of the axial Fe–(S)Met bond and the H-bond network involving Tyr67 in stabilizing the polypeptide matrix in the heme crevice. Notably, we find that the unfolding propensity and axial heme iron coordination of the present Fe–(S)Met bond-deprived variants are affected by the motional regime of the protein. In particular, electrostatic adsorption onto a negatively charged SAM surface – which would mimic the phospholipidic inner mitochondrial membrane – facilitates unfolding compared to the solution state, especially at room temperature. This finding has physiological relevance related to the cytochrome c interaction with cardiolipin at the IMM in the early stages of apoptosis. Moreover, while both immobilized variants maintain the His/OH− axial heme iron coordination up to 7 M urea, the same species in solution are subjected to urea-induced replacement of the axial hydroxide ligand by a His ligand. The contributions of the enthalpic and entropic terms to 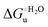 were found to be opposite (H–S compensation), indicating that the unfolding thermodynamics are strongly affected by changes in the hydrogen bonding network in the hydration sphere of the protein.
were found to be opposite (H–S compensation), indicating that the unfolding thermodynamics are strongly affected by changes in the hydrogen bonding network in the hydration sphere of the protein.
中文翻译:

Met80和Tyr67影响酵母细胞色素c的化学解折叠:比较溶液与固定状态
通过可变温度扩散循环伏安法和电子吸收,CD和MCD光谱研究了尿素诱导的酿酒酵母iso-1细胞色素c(ycc)Met80Ala和Met80Ala / Tyr67Ala变体的变性。两种变体都易于展开-以变性剂无限稀释时展开的自由能表示,
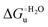 –与显示完整的Met / His配比的物种相比更大,如先前观察到的固定在功能化电极上的相同物种。这与轴向Fe–(S)Met键和涉及Tyr67的H键网络在稳定血红素缝隙中的多肽基质中的作用是一致的。值得注意的是,我们发现当前Fe–(S)Met键缺失的变异体的展开倾向和轴向血红素铁配位受蛋白质的运动方式影响。特别是,与溶液状态相比,静电吸附到带负电荷的SAM表面上(会模拟磷脂质内线粒体膜)有助于展开,特别是在室温下。这一发现具有与细胞色素c相关的生理相关性在细胞凋亡早期与IMM的心磷脂相互作用。此外,虽然两个固定的变体保持他的/ OH -轴向血红素铁协调高达7M尿素,溶液中的相同物种经受尿素诱导的替换由他的配位体的轴向氢氧化物配位体。
–与显示完整的Met / His配比的物种相比更大,如先前观察到的固定在功能化电极上的相同物种。这与轴向Fe–(S)Met键和涉及Tyr67的H键网络在稳定血红素缝隙中的多肽基质中的作用是一致的。值得注意的是,我们发现当前Fe–(S)Met键缺失的变异体的展开倾向和轴向血红素铁配位受蛋白质的运动方式影响。特别是,与溶液状态相比,静电吸附到带负电荷的SAM表面上(会模拟磷脂质内线粒体膜)有助于展开,特别是在室温下。这一发现具有与细胞色素c相关的生理相关性在细胞凋亡早期与IMM的心磷脂相互作用。此外,虽然两个固定的变体保持他的/ OH -轴向血红素铁协调高达7M尿素,溶液中的相同物种经受尿素诱导的替换由他的配位体的轴向氢氧化物配位体。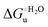 发现焓和熵项的贡献是相反的(H–S补偿),表明展开的热力学受到蛋白质水化范围内氢键网络变化的强烈影响。
发现焓和熵项的贡献是相反的(H–S补偿),表明展开的热力学受到蛋白质水化范围内氢键网络变化的强烈影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号