当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Amine-functionalized microporous covalent organic polymers for adsorptive removal of a gaseous aliphatic aldehyde mixture
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2020-09-09 , DOI: 10.1039/d0en00537a Kumar Vikrant 1, 2, 3, 4 , Yao Qu 1, 2, 3, 4 , Ki-Hyun Kim 1, 2, 3, 4 , Danil W. Boukhvalov 5, 6, 7, 8, 9 , Wha-Seung Ahn 4, 10, 11, 12
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2020-09-09 , DOI: 10.1039/d0en00537a Kumar Vikrant 1, 2, 3, 4 , Yao Qu 1, 2, 3, 4 , Ki-Hyun Kim 1, 2, 3, 4 , Danil W. Boukhvalov 5, 6, 7, 8, 9 , Wha-Seung Ahn 4, 10, 11, 12
Affiliation
|
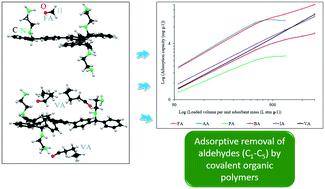
To pursue effective removal of gaseous volatile organic compounds (VOCs), adsorptive removal of a six-component aliphatic aldehyde mixture in gas phase (i.e., formaldehyde (FA), acetaldehyde (AA), propionaldehyde (PA), butyraldehyde (BA), isovaleraldehyde (IA), and valeraldehyde (VA)) was investigated using two microporous covalent organic polymers (COPs) functionalized with ethylenediamine (CBAP-1 (EDA) [CE]) and diethylenetriamine (CBAP-1 (DETA) [CD]); CBAP represents a carbonyl-incorporated aromatic polymer. A commercial activated carbon (AC) was utilized as a reference adsorbent. The gaseous aliphatic aldehyde mixture was composed of ∼1 Pa (FA/AA) and ∼0.2 Pa (PA/BA/IA/VA) with methanol (2.6 Pa) and water (15 Pa). CE displayed the highest 10% breakthrough volume (BTV10) values for FA and VA of 714 and >2400 L atm g−1, respectively. The apparent superiority of CE over CD (e.g., in terms of BTV10 values) was limited to the lighter aldehydes (FA, AA, and PA) when compared in terms of adsorption capacity values at 50% breakthrough. The adsorption capacity values (at 50% breakthrough) for CE ranged from 14 (FA) to >13 mg g−1 (VA), while those for CD were 11 (FA) to >13 (VA) mg g−1. The results of AC were far smaller at 0.003 (FA) to 7 (VA) mg g−1. Density functional theory (DFT)-based simulations indicated the non-covalent interactions between carbonyl groups present in aldehyde molecules and the amine functionalities present in COPs as the primary mechanism while the formation of a Schiff base/imine (covalent interaction) as a less favorable (secondary) pathway. In addition, the dipole–dipole interactions between the amine-functionalized COP surface and the carbonyl group of aldehydes promoted the transformation of the weak van der Waals forces into robust electrostatic interactions with the prominent appearance of such a process in heavier aldehydes. As such, the results of DFT calculations agreed well with the adsorption kinetics and material characterization data. Consequently, the COP–aldehyde interactions reflected a synergistic combination of physical and chemical adsorption. On the whole, the present study is expected to contribute to the establishment of suitable strategies for the fabrication of advanced adsorbents to effectively treat gaseous VOC mixtures under real-world conditions.
中文翻译:

胺官能化的微孔共价有机聚合物,用于吸附去除气态脂肪醛混合物
为了有效去除气态挥发性有机化合物(VOC),在气相中吸附去除六组分脂肪醛混合物(即,甲醛(FA),乙醛(AA),丙醛(PA),丁醛(BA),异戊醛(IA)和戊醛(VA))均使用两种用乙二胺(CBAP-1)功能化的微孔共价有机聚合物(COP)进行了研究(EDA)[CE])和二亚乙基三胺(CBAP-1(DETA)[CD]);CBAP代表结合有羰基的芳族聚合物。商业活性炭(AC)被用作参考吸附剂。气态脂族醛混合物由〜1 Pa(FA / AA)和〜0.2 Pa(PA / BA / IA / VA)以及甲醇(2.6 Pa)和水(15 Pa)组成。CE显示FA和VA的最高10%穿透体积(BTV10)值分别为714和> 2400 L atm g -1。CE明显优于CD(例如当以50%突破时的吸附容量值进行比较时,就BTV10值而言)仅限于较轻的醛类(FA,AA和PA)。CE的吸附容量值(在50%穿透下)范围为14(FA)至> 13 mg g -1(VA),而CD的吸附容量值为11(FA)至> 13(VA)mg g -1。AC的结果要小得多,为0.003(FA)至7(VA)mg g -1。基于密度泛函理论(DFT)的模拟表明,醛分子中存在的羰基与COP中存在的胺官能团之间的非共价相互作用是主要机理,而席夫碱/亚胺的形成(共价相互作用)则较不利(次要)途径。此外,胺官能化的COP表面与醛的羰基之间的偶极相互作用使该反应的弱范德华力转化为强力的静电相互作用,这种过程在较重的醛中表现突出。因此,DFT计算的结果与吸附动力学和材料表征数据非常吻合。因此,COP-醛相互作用反映了物理和化学吸附的协同作用。
更新日期:2020-11-03
中文翻译:

胺官能化的微孔共价有机聚合物,用于吸附去除气态脂肪醛混合物
为了有效去除气态挥发性有机化合物(VOC),在气相中吸附去除六组分脂肪醛混合物(即,甲醛(FA),乙醛(AA),丙醛(PA),丁醛(BA),异戊醛(IA)和戊醛(VA))均使用两种用乙二胺(CBAP-1)功能化的微孔共价有机聚合物(COP)进行了研究(EDA)[CE])和二亚乙基三胺(CBAP-1(DETA)[CD]);CBAP代表结合有羰基的芳族聚合物。商业活性炭(AC)被用作参考吸附剂。气态脂族醛混合物由〜1 Pa(FA / AA)和〜0.2 Pa(PA / BA / IA / VA)以及甲醇(2.6 Pa)和水(15 Pa)组成。CE显示FA和VA的最高10%穿透体积(BTV10)值分别为714和> 2400 L atm g -1。CE明显优于CD(例如当以50%突破时的吸附容量值进行比较时,就BTV10值而言)仅限于较轻的醛类(FA,AA和PA)。CE的吸附容量值(在50%穿透下)范围为14(FA)至> 13 mg g -1(VA),而CD的吸附容量值为11(FA)至> 13(VA)mg g -1。AC的结果要小得多,为0.003(FA)至7(VA)mg g -1。基于密度泛函理论(DFT)的模拟表明,醛分子中存在的羰基与COP中存在的胺官能团之间的非共价相互作用是主要机理,而席夫碱/亚胺的形成(共价相互作用)则较不利(次要)途径。此外,胺官能化的COP表面与醛的羰基之间的偶极相互作用使该反应的弱范德华力转化为强力的静电相互作用,这种过程在较重的醛中表现突出。因此,DFT计算的结果与吸附动力学和材料表征数据非常吻合。因此,COP-醛相互作用反映了物理和化学吸附的协同作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号