当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Relationships between Bond Strength and Spectroscopic Quantities in H-Bonds and Related Halogen, Chalcogen, and Pnicogen Bonds.
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-09-08 , DOI: 10.1021/acs.jpca.0c05936 Jia Lu 1 , Steve Scheiner 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-09-08 , DOI: 10.1021/acs.jpca.0c05936 Jia Lu 1 , Steve Scheiner 1
Affiliation
|
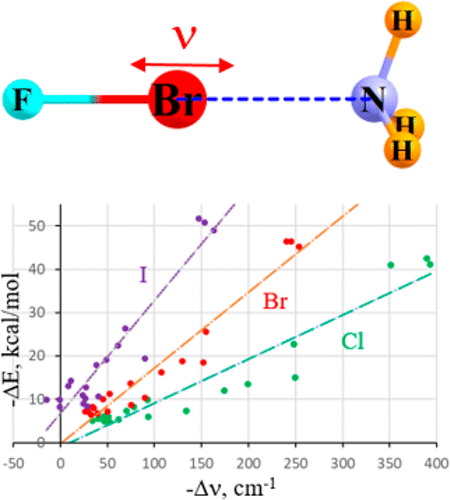
A large number of systems were studied that contain either a H-bond (HB) or the closely related halogen (X), chalcogen (Y), or pnicogen (Z) bond. Many of the same relationships between the strength of the HB and spectroscopic parameters apply as well to the other noncovalent bonds. There is a very nearly linear relationship between the interaction energy and the red shift that occurs within the stretching frequency of the pertinent F–A covalent bond of the Lewis acid (where A refers to H, X, Y, or Z). This vibrational mode also undergoes an intensification in all of these bond types, but this quantity is slightly less indicative of the bond strength for XB, YB, and ZB than it is for HB. On the other hand, most of the various noncovalent bonds display a better relationship between energetics and NMR chemical shifts than do the H-bonds, highly auspicious for the use of NMR spectra in diagnosing the presence and strengths of these bonds. An important element in NMR chemical shifts is the internal geometry change induced by complexation.
中文翻译:

氢键的键强度与光谱数量之间的关系以及相关的卤素键,硫属元素和Pnicogen键。
研究了大量包含H键(HB)或密切相关的卤素(X),硫属元素(Y)或Pnicogen(Z)键的系统。HB强度与光谱参数之间的许多相同关系也适用于其他非共价键。在路易斯酸的相关F–A共价键的拉伸频率范围内(其中A表示H,X,Y或Z),相互作用能与红移之间存在非常接近的线性关系。在所有这些键类型中,该振动模式也会经历增强,但是与HB相比,此量略小于XB,YB和ZB的键强度。另一方面,与H键相比,大多数非共价键在能量学和NMR化学位移之间表现出更好的关系,对于使用NMR光谱诊断这些键的存在和强度,我们表示非常感谢。NMR化学位移中的一个重要元素是络合引起的内部几何形状变化。
更新日期:2020-09-24
中文翻译:

氢键的键强度与光谱数量之间的关系以及相关的卤素键,硫属元素和Pnicogen键。
研究了大量包含H键(HB)或密切相关的卤素(X),硫属元素(Y)或Pnicogen(Z)键的系统。HB强度与光谱参数之间的许多相同关系也适用于其他非共价键。在路易斯酸的相关F–A共价键的拉伸频率范围内(其中A表示H,X,Y或Z),相互作用能与红移之间存在非常接近的线性关系。在所有这些键类型中,该振动模式也会经历增强,但是与HB相比,此量略小于XB,YB和ZB的键强度。另一方面,与H键相比,大多数非共价键在能量学和NMR化学位移之间表现出更好的关系,对于使用NMR光谱诊断这些键的存在和强度,我们表示非常感谢。NMR化学位移中的一个重要元素是络合引起的内部几何形状变化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号