当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Syntheses of (Z)-6'-Boryl-anti-1,2-oxaborinan-3-enes via a Dienylboronate Protoboration and Asymmetric Allylation Reaction Sequence.
Organic Letters ( IF 4.9 ) Pub Date : 2020-09-09 , DOI: 10.1021/acs.orglett.0c02657 Jichao Chen 1 , Ming Chen 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-09-09 , DOI: 10.1021/acs.orglett.0c02657 Jichao Chen 1 , Ming Chen 1
Affiliation
|
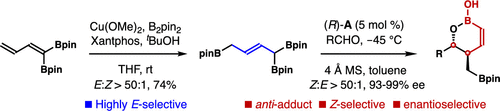
The enantioselective synthesis of 6′-boryl-anti-1,2-oxaborinan-3-enes is reported. A Cu-catalyzed highly stereoselective 1,4-protoboration of 1,1-bisboryl-1,3-butadiene is developed to generate (E)-α,δ-bisboryl-crotylboronate. The chiral phosphoric-acid-catalyzed asymmetric allylboration of aldehydes with the boron reagent produces 6′-boryl-anti-1,2-oxaborinan-3-enes with excellent Z-selectivities and enantioselectivities. The product contains a vinyl and alkyl boronate unit that can directly participate in a variety of subsequent transformations.
中文翻译:

(Z)-6'-硼基-抗-1,2-氧杂硼酸-3-烯的对映选择性合成通过二烯基硼酸酯的原硼酸酯化和不对称的烯丙基化反应序列进行。
6'-boryl-的对映选择性合成的抗-报道1,2-氧杂硼环己烷3 -烯。开发了Cu催化的1,1-双硼基-1,3-丁二烯的高立体选择性1,4-硼氢化,生成(E)-α,δ-双硼基-巴豆基硼酸酯。与硼试剂醛的手性磷酸的催化不对称allylboration产生6'- boryl-反- 1,2-二氧杂硼环己烷3 -烯具有优异的Z-选择性和对映选择性。该产品包含乙烯基和烷基硼酸酯单元,可以直接参与各种后续转化。
更新日期:2020-09-20
中文翻译:

(Z)-6'-硼基-抗-1,2-氧杂硼酸-3-烯的对映选择性合成通过二烯基硼酸酯的原硼酸酯化和不对称的烯丙基化反应序列进行。
6'-boryl-的对映选择性合成的抗-报道1,2-氧杂硼环己烷3 -烯。开发了Cu催化的1,1-双硼基-1,3-丁二烯的高立体选择性1,4-硼氢化,生成(E)-α,δ-双硼基-巴豆基硼酸酯。与硼试剂醛的手性磷酸的催化不对称allylboration产生6'- boryl-反- 1,2-二氧杂硼环己烷3 -烯具有优异的Z-选择性和对映选择性。该产品包含乙烯基和烷基硼酸酯单元,可以直接参与各种后续转化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号